Supplementary information: Test methods used during development or manufacture: Tablet friability
This text is based on the internationally-harmonized texts developed by the Pharmacopoeial Discussion Group (PDG). Some editorial modifications have been made in order to be in line with the style used in The International Pharmacopoeia.
This method text provides guidance for the friability determination of compressed, uncoated tablets. The test procedure presented is generally applicable to most compressed tablets. Measurement of tablet friability supplements other physical strength measurements such as tablet-breaking force.
Use a drum with an internal diameter between 283–291 mm and a depth between 36–40 mm, of transparent synthetic polymer with polished internal surfaces and subject to minimum static build-up (see Figure 1 for a typical apparatus). One side of the drum is removable. The tablets are tumbled at each turn of the drum by a curved projection with an inside radius between 75.5–85.5 mm that extends from the middle of the drum to the outer wall. The outer diameter of the central ring is between 24.5–25.5 mm. The drum is attached to the horizontal axis of a device that rotates at 25 ± 1 rpm. Thus, at each turn the tablets roll or slide and fall onto the drum wall or on to each other.
For tablets with a unit weight equal to or less than 650 mg, take a sample of whole tablets n corresponding as near as possible to 6.5 g. For tablets with a unit weight of more than 650 mg, take a sample of 10 whole tablets. The tablets should be carefully dedusted prior to testing. Accurately weigh the tablet sample and place the tablets in the drum. Rotate the drum 100 times and remove the tablets. Remove any loose dust from the tablets as before and accurately weigh.
Generally the test is run once. If obviously cracked, cleaved or broken tablets are present in the tablet sample after tumbling the sample fails the test. If the results are difficult to interpret or if the weight loss is greater than the targeted value the test should be repeated twice and the mean of the three tests determined. A maximum weight loss (obtained from a single test or from the mean of three tests) of not more than 1.0% is considered acceptable for most products.
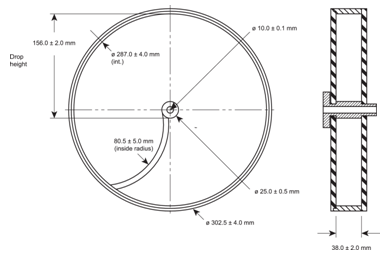
Figure 1. Tablet friability apparatus 1
1 Reproduced with the kind permission of the European Directorate for the Quality of Medicines & HealthCare, Council of Europe, Strasbourg, France.
If tablet size or shape causes irregular tumbling adjust the drum base so that the base forms an angle of about 10º with the horizontal and the tablets no longer bind together when lying next to each other, which prevents them from falling freely.
Effervescent tablets and chewable tablets may have different specifications as far as friability is concerned. In the case of hygroscopic tablets an appropriate humidity-controlled environment is required for testing.
Drums with dual scooping projections, or apparatus with more than one drum for the running of multiple samples at one time, are also permitted.