Monographs: Pharmaceutical substances: Dopamine hydrochloride (Dopamini hydrochloridum)
Molecular formula. C8H11NO2,HCl
Relative molecular mass. 189.6
Graphic formula.
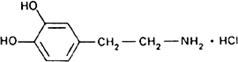
Chemical name. 4-(2-Aminoethyl)pyrocatechol hydrochloride; 4-(2-amino-ethyl)-1,2-benzenediol hydrochloride; CAS Reg. No. 62-31-7.
Description. Colourless crystals or a white or almost white, crystalline powder; odourless.
Solubility. Freely soluble in water; soluble in methanol R; practically insoluble in ether R and toluene R.
Category. Cardiovascular drug; sympathomimetic.
Storage. Dopamine hydrochloride should be kept in a well-closed container, protected from light.
Requirements
Definition. Dopamine hydrochloride contains not less than 98.0% and not more than 101.0% of C8H11NO2,HCl, calculated with reference to the dried substance.
Identity tests
• Either tests A and D or tests B, C and D may be applied.
A. Carry out the examination as described under 1.7 Spectrophotometry in the infrared region. The infrared absorption spectrum is concordant with the spectrum obtained from dopamine hydrochloride RS or with the reference spectrum of dopamine hydrochloride.
B. The absorption spectrum of a 0.020 mg/mL solution in hydrochloric acid (0.1 mol/l) VS, when observed between 230 nm and 350 nm, exhibits a maximum at about 280 nm and a minimum at about 249 nm; the absorbance of a 1-cm layer at 280 nm is about 0.54.
C. Dissolve 0.05 g in 5 mL of water and add while stirring 10 mL of 4-amino-antipyrine TS2; a red colour is produced.
D. A 20 mg/mL solution yields reaction A described under 2.1 General identification tests as characteristic of chlorides.
Heavy metals. Use 1.0 g for the preparation of the test solution as described under 2.2.3 Limit test for heavy metals, Procedure 1; determine the heavy metals content according to Method A; not more than 20 μg/g.
Clarity and colour of solution. A solution of 1.0 g in 10 mL of water is clear and colourless.
Sulfated ash. Not more than 1.0 mg/g.
Loss on drying. Dry to constant weight at 105°C; it loses not more than 5.0 mg/g.
Related substances. Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography using silica gel R1 as the coating substance and a mixture of 13 volumes of chloroform R, 9 volumes of methanol R, and 4 volumes of acetic acid (~300 g/l) TS as the mobile phase. Apply separately to the plate 10 μl of each of 2 solutions in methanol R containing (A) 30 mg of the test substance per mL and (B) 0.3 mg of dopamine hydrochloride RS per mL. After removing the plate from the chromatographic chamber, allow it to dry at room temperature for several minutes, then spray it evenly with a freshly prepared mixture of 2 volumes of ferric chloride (50 g/l) TS and 1 volume of potassium ferricyanide (50 g/l) TS. Examine the chromatogram in daylight. Apart from the principal spot, not more than 3 spots are obtained with solution A, and the estimated sum of impurities is not larger than that estimated from the chromatogram obtained with solution B.
Assay. In order to avoid overheating in the reaction medium, mix thoroughly throughout the titration and stop the titration immediately after the end-point has been reached.
Dissolve 0.150 g in 10 mL of anhydrous formic acid R and add 50 mL of acetic anhydride R. Carry out a potentiometric titration using perchloric acid (0.1 mol/L) VS, as described under 2.6 Non-aqueous titration.
1 mL of 0.1 M perchloric acid is equivalent to 18.96 mg of C8H11NO2,HCl.
Additional requirements for Dopamine hydrochloride for parenteral use
Complies with the monograph for "Parenteral preparations".
Bacterial endotoxins. Carry out the test as described under 3.4 Test for bacterial endotoxins; contains not more than 16.67 IU of endotoxin RS per mg.