Monographs: Pharmaceutical substances: Edrophonium chloride (Edrophonii chloridum)
Molecular formula. C10H16ClNO
Relative molecular mass. 201.7
Graphic formula.
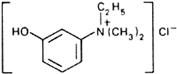
Chemical name.
Ethyl(m-hydroxyphenyl)dimethylammonium chloride; N-ethyl-3-hydroxy-N,N-dimethylbenzenaminium chloride; CAS Reg. No. 116-38-1.
Description. A white, crystalline powder; odourless.
Solubility. Soluble in 0.5 parts of water and in 5 parts of ethanol (~750 g/l) TS; practically insoluble in ether R.
Category. Diagnostic agent.
Storage. Edrophonium chloride should be kept in a well-closed container, protected from light.
Requirements
Definition. Edrophonium chloride contains not less than 98.5% and not more than 101.0% of C10H16ClNO, calculated with reference to the dried substance.
Identity tests
A. The absorption spectrum of a 0.050 mg/mL solution in hydrochloric acid (0.1 mol/l) VS, when observed between 230 nm and 350 nm, exhibits a maximum at about 273 nm; the absorbance of a 1-cm layer at this wavelength is about 0.55.
B. The absorption spectrum of a 10 μg/mL solution in sodium hydroxide (0.1 mol/l) VS, when observed between 230 nm and 350 nm, exhibits maxima at about 240 nm and 294 nm; the absorbances of a 1-cm layer at these wavelengths are about 0.55 and 0.17, respectively.
C. Dissolve 0.05 g in 2 mL of water and add 0.05 mL of ferric chloride (25 g/l) TS; a reddish violet colour is produced.
D. Melting temperature, about 168°C with decomposition.
E. A 20 mg/mL solution yields reaction A described under 2.1 General identification tests as characteristic of chlorides.
Sulfated ash. Not more than 1.0 mg/g.
Loss on drying. Dry at ambient temperature under reduced pressure (not exceeding 0.6 kPa or about 5 mm of mercury) over phosphorus pentoxide R for 24 hours; it loses not more than 5.0 mg/g.
pH value. pH of a 0.10 g/mL solution, 4.0-5.0.
Dimethylaminophenol. Dissolve 0.1 g in 10 mL of water, add 5 mL of phosphate buffer, pH 8.0, TS, and extract with 2 quantities, each of 20 mL of chloroform R.
Wash the extracts successively with 2 quantities, each of 10 mL of water, and extract with 10 mL of sodium hydroxide (0.1 mol/l) VS. Measure the absorbance of the sodium hydroxide extract using a 1-cm layer at 293 nm; not greater than 0.25.
Assay. In order to avoid overheating in the reaction medium, mix thoroughly throughout the titration and stop the titration immediately after the end-point has been reached.
Dissolve 0.150 g in 60 mL of a mixture of equal volumes of acetic anhydride R and anhydrous acetic acid R. Carry out a potentiometric titration using perchloric acid (0.1 mol/L) VS, as described under 2.6 Non-aqueous titration.
1 mL of perchloric acid (0.1 mol/L) VS is equivalent to 20.17 mg of C10H16ClNO.