Monographs: Pharmaceutical substances: Emtricitabine (Emtricitabinum)
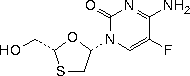
C8H10FN3O3S
Relative molecular mass. 247.2
Chemical name. 4-amino-5-fluoro-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2(1H)-one; 5-fluoro-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine; (-)-2',3'-dideoxy-5-fluoro-3'-thiacytidine; CAS Reg. N° 143491-57-0.
Description. White to almost white, crystalline powder.
Solubility. Freely soluble in methanol R and water R, practically insoluble in dichloromethane R.
Category. Antiretroviral (Nucleoside Reverse Transcriptase Inhibitor).
Storage. Emtricitabine should be kept in a tightly closed container.
Additional information. Emtricitabine may exhibit polymorphism.
Requirements
Definition. Emtricitabine contains not less than 99.0% and not more than 101.0% of emtricitabine (C8H10FN3O3S), calculated with reference to the dried substance.
Manufacture. The production method is validated to ensure that the substance, if tested, would comply with a limit of not more than 0.3% for the (2S,5R)-enantiomer using a suitable chiral chromatographic method.
Identity tests
• Either tests A, B and D or tests C and D may be applied.
A. Carry out test A.1 or, where UV detection is not available, test A.2.
A.1 Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography, using silica gel R6 as the coating substance and a mixture of 90 volumes of dichloromethane R, 10 volumes of methanol R and 3 volumes of glacial acetic acid R as the mobile phase. Apply separately to the plate 5 μl of the following two solutions in methanol R. For solution (A) use 5 mg of the test substance per mL. For solution (B) use 5 mg of emtricitabine RS per mL. After removing the plate from the chromatographic chamber, allow it to dry exhaustively in air or in a current of cool air. Examine the chromatogram in ultraviolet light (254 nm).
The principal spot obtained with solution A corresponds in position, appearance, and intensity with that obtained with solution B.
A.2 Carry out the test as descried under 1.14.1 Chromatography, Thin-layer chromatography, using the conditions described under test A.1 but using silica gel R5 as the coating substance. Stain the plate with iodine vapor and examine in daylight.
The principal spot obtained with solution A corresponds in position, appearance, and intensity with that obtained with solution B.
B. The absorption spectrum (1.6) of a 20 µg/mL solution, when observed between 220 nm and 350 nm, exhibits two maxima at about 237 nm and 281 nm; the specific absorbance (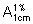 ) is 325 to 355 and 340 to 370 respectively.
) is 325 to 355 and 340 to 370 respectively.
C. Carry out the examination as described under 1.7 Spectrophotometry in the infrared region. The infrared absorption spectrum is concordant with the spectrum obtained from emtricitabine RS or with the reference spectrum of emtricitabine.
If the spectra thus obtained are not concordant, repeat the test using the residues obtained by separately dissolving the test substance and emtricitabine RS in a small amount of methanol R and evaporating to dryness. The infrared absorption spectrum is concordant with the spectrum obtained from emtricitabine RS.
D. Determine the specific optical rotation (1.4) using a 2.5 mg/mL solution in water R and calculate with reference to the dried substance; 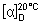 = -105.0° to -115.0°.
= -105.0° to -115.0°.
Loss on drying. Dry for 3 hours at 105 °C; it loses not more than 5 mg/g.
Heavy metals. Use 1.0 g for the preparation of the test solution as described under 2.2.3 Limit test for heavy metals, Procedure 1; determine the heavy metals content according to Method A; not more than 20 μg/g.
Sulfated ash (2.3). Not more than 1.0 mg/g.
Related substances. Carry out the test as described under 1.14.1 Chromatography, High-performance liquid chromatography, using a stainless steel column (25 cm x 4.6 mm) packed with base deactivated particles of silica gel the surface of which has been modified with chemically bonded octadecylsilyl groups (5 μm).
Use the following conditions for gradient elution:
Mobile phase A: 5 volumes of phosphate buffer and 95 volumes of water R.
Mobile phase B: 70 volumes of acetonitrile R, 5 volumes of phosphate buffer and 25 volumes of water R.
Prepare the phosphate buffer by dissolving 27.22 g of potassium dihydrogen phosphate R in 1000 mL of water R.
|
Time |
Mobile phase A |
Mobile phase B |
Comments |
|
0 – 9 |
93 |
7 |
Isocratic |
|
9 – 15 |
93 to 0 |
7 to 100 |
Linear gradient |
|
15 – 19 |
0 |
100 |
Isocratic |
|
19 – 19.1 |
0 to 93 |
100 to 7 |
Return to initial composition |
|
19.1 - 30 |
93 |
7 |
Re-equilibration |
Prepare the following solutions in water R. For solution (1) use 0.5 mg of the test substance per mL. For solution (2) dilute a suitable volume of solution (1) to obtain a concentration equivalent to 0.5 μg of emtricitabine per mL.
For the system suitability test: prepare solution (3) using 5 mL of solution (1) and 2 mL of phosphoric acid (~105 g/l) TS, heat carefully in a boiling water-bath for 15 minutes.
Operate with a flow rate of 1.0 mL per minute. As a detector use an ultraviolet spectrophotometer set at a wavelength of 280 nm.
Maintain the column temperature at 35 °C.
Inject 20 μl of solution (3). The test is not valid unless the resolution between the peak due to emtricitabine (retention time about 9 minutes) and the peak with a relative retention of about 1.3 is not less than 6.
Inject alternatively 20 μl each of solutions (1) and (2).
In the chromatogram obtained with solution (1) the area of any peak eluting before the principal peak is not greater than 5 times the area of the principal peak in the chromatogram obtained with solution (2) (0.5%); the area of not more than two such peaks is greater than the area of the principal peak in the chromatogram obtained with solution (2) (0.1%); the area of any peak eluting after the principal peak is not greater than 7 times the area of the principal peak in the chromatogram obtained with solution (2) (0.7%); the area of not more than two such peaks is greater than the area of the principal peak in the chromatogram obtained with solution (2) (0.1%). The sum of the areas of all peaks, other than the principal peak is not greater than 10 times the area of the principal peak in the chromatogram obtained with solution (2) (1%). Disregard any peak with an area less than 0.5 times the area of the principal peak in the chromatogram obtained with solution (2) (0.05%).
Assay
Dissolve 0.15 g, accurately weighed, in 40 mL of glacial acetic acid R1 and titrate with perchloric acid (0.1 mol/l) VS, determining the end-point potentiometrically as described under 2.6 Non-aqueous titration Method A.
Each mL of perchloric acid (0.1 mol/l) VS is equivalent to 24.73 mg of C8H10FN3O3S, calculated with reference to the dried substance.
Impurities
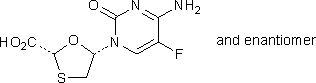
A. (2RS,5SR)-5-(4-amino-5-fluoro-2-oxopyrimidin-1(2H)-yl)-1,3-oxathiolan-2-carboxylic acid,
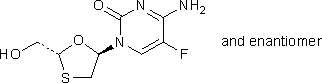
B. 4-amino-5-fluoro-1-[(2RS,5RS)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2(1H)-one (racemic trans-emtricitabine),
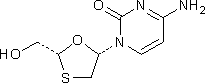
C. 4-amino-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2(1H)-one (lamivudine),
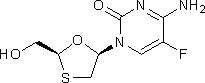
D. 4-amino-5-fluoro-1-[(2S,5R)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2(1H)-one (emtricitabine enantiomer),
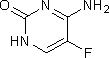
E. 4-amino-5-fluoropyrimidin-2(1H)-one (5-fluorocytosine),
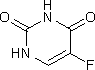
F. 5-fluoropyrimidin-2,4(1H,3H)-dione (5-fluorouracil),
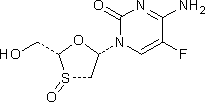
G. 4-amino-5-fluoro-1-[(2R,3S,5S)-2-(hydroxymethyl)-3-oxo-1,3-oxa-λ4-thiolan-5-yl]pyrimidin-2(1H)-one (emtricitabine S-oxide (S)-epimer),

H. 4-amino-5-fluoro-1-[(2R,3R,5S)-2-(hydroxymethyl)-3-oxo-1,3-oxa-λ4-thiolan-5-yl]pyrimidin-2(1H)-one (emtricitabine S-oxide (R)-epimer),
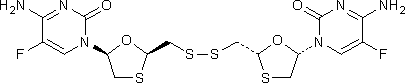
I. 1,1'-[disulfanediylbis(methylene-(2R,5S)-1,3-oxathiolane-2,5-diyl)]bis(4-amino-5-fluoropyrimidin-2(1H)-one),
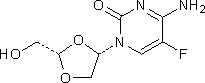
J. 4-amino-5-fluoro-1-[(2S,4S)-2-(hydroxymethyl)-1,3-dioxolan-4-yl]pyrimidin-2(1H)-one.