Monographs: Pharmaceutical substances: Ephedrine (Ephedrinum)
Ephedrine, anhydrous
Ephedrine hemihydrate
Molecular formula. C10H15NO (anhydrous); C10H15NO, 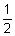 H2O (hemihydrate).
H2O (hemihydrate).
Relative molecular mass. 165.2 (anhydrous); 174.2 (hemihydrate).
Graphic formula.
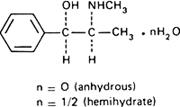
Chemical name. (-)-Ephedrine; [R-(R*,S*)]-α-[1-(methylamino)ethyl]benzenemethanol; CAS Reg. No. 299-42-3 (anhydrous).
(-)-Ephedrine hemihydrate; [R-(R*,S*)]-α-[1-(methylamino)ethyl]benzenemethanol hemihydrate; CAS Reg. No. 50906-05-3 (hemihydrate).
Description. Colourless crystals or a white, crystalline powder; odourless or with a slight, aromatic odour.
Solubility. Soluble in water; very soluble in ethanol (~750 g/l) TS; freely soluble in ether R.
Category. Antiasthmatic drug.
Storage. Ephedrine should be kept in a well-closed container, protected from light.
Labelling. The designation on the container should state whether the substance is the hemihydrate or is in the anhydrous form.
Additional information. Solutions of Ephedrine in chloroform R may become turbid, especially when the substance contains more than 10 mg of water per g. Even in the absence of light, Ephedrine is gradually degraded on exposure to a humid atmosphere, the decomposition being faster at higher temperatures. Anhydrous Ephedrine melts at about 38°C, whereas Ephedrine hemihydrate melts at about 42°C.
Requirements
Definition. Ephedrine contains not less than 98.5% and not more than 101.0% of C10H15NO, calculated with reference to the anhydrous substance.
Identity tests
A. The absorption spectrum of a 0.50 mg/mL solution in hydrochloric acid (0.1 mol/l) VS, when observed between 230 nm and 350 nm, exhibits 3 maxima at about 251 nm, 257 nm, and 263 nm.
B. Dissolve 10 mg in 1 mL of water and add 0.1 mL of copper(II) sulfate (80 g/l) TS, followed by 2 mL of sodium hydroxide (~80 g/l) TS; a violet colour is produced. Add 1 mL of ether R and shake; a purple colour is produced in the ethereal layer and a blue colour in the aqueous layer.
C. Dissolve 0.05 g in 5 mL of water. Add a few drops of sodium hydroxide (~80 g/l) TS and 4 mL of potassium ferricyanide (50 g/l) TS, and heat; an odour of benzaldehyde is perceptible.
Specific optical rotation. Dissolve about 2.25 g, accurately weighed, in 15 mL of hydrochloric acid (~70 g/l) TS and dilute to 50 mL with water. Calculate the result with reference to the anhydrous substance;  = -41° to -43°
= -41° to -43°
Chlorides. Dissolve 0.70 g in a mixture of 2 mL of nitric acid (~130 g/l) TS and 20 mL of water, and proceed as described under 2.2.1 Limit test for chlorides; the chloride content is not more than 0.35 mg/g.
Sulfates. Dissolve 1.2 g in 20 mL of water and proceed as described under 2.2.2 Limit test for sulfates; the sulfate content is not more than 0.4 mg/g.
Sulfated ash. Not more than 1.0 mg/g.
Water. Determine as described under 2.8 Determination of water by the Karl Fischer method, Method A. For the anhydrous form use about 2 g of the substance; the water content is not more than 10 mg/g. For the hemihydrate use about 1 g of the substance; the water content is not less than 45 mg/g and not more than 55 mg/g.
Assay. Dissolve about 0.5 g, accurately weighed, in 5 mL of ethanol (~750 g/l) TS, add 50.0 mL of hydrochloric acid (0.1 mol/l) VS and titrate with sodium hydroxide (0.1 mol/l) VS, using methyl red/ethanol TS as indicator. Each mL of hydrochloric acid (0.1 mol/l) VS is equivalent to 16.52 mg of C10H15NO.