Monographs: Pharmaceutical substances: Ephedrine hydrochloride (Ephedrini hydrochloridum)
Molecular formula. C10H15NO,HCl
Relative molecular mass. 201.7
Graphic formula.
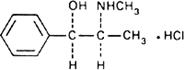
Chemical name. (-)-Ephedrine hydrochloride; [R-(R*,S*)]-α-[1-(methylamino)ethyl]benzenemethanol hydrochloride; CAS Reg. No. 50-98-6.
Description. Colourless crystals or a white, crystalline powder; odourless.
Solubility. Soluble in 4 parts of water; soluble in ethanol (~750 g/l) TS; practically insoluble in ether R.
Category. Antiasthmatic drug.
Storage. Ephedrine hydrochloride should be kept in a well-closed container, protected from light.
Additional information. Ephedrine hydrochloride darkens on exposure to light.
Requirements
Definition. Ephedrine hydrochloride contains not less than 99.0% and not more than 101.0% of C10H15NO,HCl, calculated with reference to the dried substance.
Identity tests
A. The absorption spectrum of a 0.50 mg/mL solution, when observed between 230 nm and 350 nm, exhibits maxima at about 251 nm, 257 nm, and 263 nm; the absorbances of a 1-cm layer at these wavelengths are about 0.37, 0.48, and 0.36, respectively.
B. Dissolve 10 mg in 1 mL of water and add 0.1 mL of copper(II) sulfate (80 g/l) TS, followed by 2 mL of sodium hydroxide (~80 g/l) TS; a violet colour is produced. Add 1 mL of ether R and shake; a purple colour is produced in the ethereal layer and a blue colour in the aqueous layer.
C. Dissolve 0.05 g in 5 mL of water. Add a few drops of sodium hydroxide (~80 g/l) TS and 4 mL of potassium ferricyanide (50 g/l) TS, and heat; an odour of benzaldehyde is perceptible.
D. A 0.05 g/mL solution yields reaction A described under 2.1 General identification tests as characteristic of chlorides.
Melting range. 217-220°C.
Specific optical rotation. Use a 50 mg/mL solution;  = -33.0° to -35.5°.
= -33.0° to -35.5°.
Sulfates. Dissolve 0.050 g in 40 mL of water and add 1.5 mL of hydrochloric acid (~70 g/l) TS and 1 mL of barium chloride (50 g/l) TS; no turbidity develops within 10 minutes.
Clarity and colour of solution. A solution of 1.0 g in 10 mL of water is clear, or not more opalescent than opalescence standard TS2, and colourless.
Sulfated ash. Not more than 1.0 mg/g.
Loss on drying. Dry to constant weight at 105°C; it loses not more than 5.0 mg/g.
Acidity and alkalinity. Dissolve 1.0 g in 10 mL of water and add 0.1 mL of methyl red/ethanol TS; not more than 0.1 mL of sodium hydroxide (0.1 mol/l) VS or 0.1 mL of hydrochloric acid (0.1 mol/l) VS is required to obtain the midpoint of the indicator (orange).
Assay. Dissolve 0.150 g in 50 mL of dehydrated ethanol R and add 5.0 mL of hydrochloric acid (0.01 mol/L) VS. Carry out a potentiometric titration using sodium hydroxide (0.1 mol/L) VS, as described under 2.6 Non-aqueous titration. Read the volume added between the two points of inflexion.
1 mL of sodium hydroxide (0.1 mol/L) VS is equivalent to 20.17 mg of C10H15NO,HCl.