Monographs: Pharmaceutical substances: Fludrocortisone acetate (Fludrocortisoni acetas)
Molecular formula. C23H31FO6
Relative molecular mass. 422.5
Graphic formula.
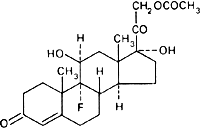
Chemical name. 9-Fluoro-11β,17,21-trihydroxypregn-4-ene-3,20-dione 21-acetate; 21-(acetyloxy)-9-fluoro-11β,17-dihydroxypregn-4-ene-3,20-dione; CAS Reg. No. 514-36-3.
Description. A white or almost white, crystalline powder; odourless or almost odourless.
Solubility. Practically insoluble in water; sparingly soluble in ethanol (~750 g/l) TS; slightly soluble in ether R.
Category. Adrenal hormone.
Storage. Fludrocortisone acetate should be kept in a well-closed container, protected from light.
Additional information. Fludrocortisone acetate is hygroscopic.
Requirements
Definition. Fludrocortisone acetate contains not less than 96.0% and not more than 104.0% of C23H31FO6, calculated with reference to the dried substance.
Identity tests
• Either test A alone or tests B and C may be applied.
A. Carry out the examination as described under 1.7 Spectrophotometry in the infrared region. The infrared absorption spectrum is concordant with the spectrum obtained from fludrocortisone acetate RS or with the reference spectrum of fludrocortisone acetate.
B. Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography, using kieselguhr R1 as the coating substance and a mixture of 10 volumes of formamide R and 90 volumes of acetone R to impregnate the plate, dipping it about 5 mm into the liquid. After the solvent has reached a height of at least 16 cm, remove the plate from the chromatographic chamber and allow it to stand at room temperature until the solvent has completely evaporated. Use the impregnated plate within 2 hours and carry out the chromatography in the same direction as the impregnation. As the mobile phase, use a mixture of 75 volumes of toluene R and 25 volumes of chloroform R. Apply separately to the plate 2 μl of each of 2 solutions in a mixture of 9 volumes of chloroform R and 1 volume of methanol R containing (A) 2.5 mg of the test substance per mL and (B) 2.5 mg of fludrocortisone acetate RS per mL. Develop the plate for a distance of 15 cm. After removing the plate from the chromatographic chamber, allow it to dry in air until the solvents have evaporated, heat it at 120°C for 15 minutes, spray it with sulfuric acid/ethanol TS, and then heat it at 120°C for 10 minutes. Allow it to cool, and examine the chromatogram in daylight and in ultraviolet light (365 nm). The principal spot obtained with solution A corresponds in position, appearance, and intensity with that obtained with solution B.
C. Heat 0.5 mL of chromic acid TS in a small test-tube in a water-bath for 5 minutes; the solution wets the sides of the tube but there is no greasiness. Add about 3 mg of the test substance and again heat in a water-bath for 5 minutes; the solution no longer wets the sides of the tube.
Specific optical rotation. Use a 10 mg/mL solution in dioxan R;  = +148° to +156°.
= +148° to +156°.
Sulfated ash. Not more than 1.0 mg/g.
Loss on drying. Dry to constant weight at 105°C; it loses not more than 10 mg/g.
Ultraviolet absorption. Absorbance of a 1-cm layer of a 10 μg/mL solution in dehydrated ethanol R at about 240 nm; 0.39-0.42.
Related substances. Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography using silica gel R2 as the coating substance and a mixture of 95 volumes of dichloroethane R, 5 volumes of methanol R, and 0.2 volumes of water as the mobile phase. Apply separately to the plate 1 μl of each of 2 solutions in a mixture of 9 volumes of chloroform R and 1 volume of methanol R containing (A) 15 mg of the test substance per mL and (B) 0.30 mg of the test substance per mL. After removing the plate from the chromatographic chamber allow it to dry in air until the solvents have evaporated; then heat it at 105°C for 10 minutes, allow it to cool, and examine the chromatogram in ultraviolet light (254 nm). Any spot obtained with solution A, other than the principal spot, is not more intense than that obtained with solution B.
Assay
• The solutions must be protected from light throughout the assay.
Dissolve about 25 mg, accurately weighed, in sufficient aldehyde-free ethanol (~750 g/l) TS to produce 250 mL. Dilute 10 mL of this solution with sufficient aldehyde-free ethanol (~750 g/l) TS to produce 50 mL. Transfer 10.0 mL of the diluted solution to a 25-mL volumetric flask, add 2.0 mL of blue tetrazolium/ethanol TS, and displace the air in the flask with oxygen-free nitrogen R. Immediately add 2.0 mL of tetramethylammonium hydroxide/ethanol TS and again displace the air with oxygen-free nitrogen R. Stopper the flask, mix the contents by gentle swirling, and allow to stand for 1 hour in a water-bath at 30 °C. Cool rapidly, add sufficient aldehyde-free ethanol (~750 g/l) TS to produce 25 mL and mix. Measure the absorbance of a 1-cm layer at the maximum at about 525 nm against a solvent cell containing a solution prepared by treating 10 mL of aldehyde-free ethanol (~750 g/l) TS in a similar manner. Calculate the amount of C23H31FO6 in the substance being tested by comparison with fludrocortisone acetate RS, similarly and concurrently examined.