Monographs: Pharmaceutical substances: Fluorescein sodium (Fluoresceinum natricum)
Molecular formula. C20H10Na2O5
Relative molecular mass. 376.3
Graphic formula.
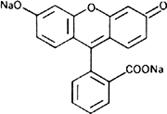
Chemical name. Fluorescein disodium salt; 2-(6-hydroxy-3-oxo-3H-xanthen-9-yl)benzoic acid disodium salt; 3',6'-dihydroxyspiro[isobenzofuran-1(3H),9'-[9H]xanthene]-3-one disodium salt; CAS Reg. No. 518-47-8.
Description. An orange-red powder; odourless.
Solubility. Soluble in 1.5 parts of water; soluble in ethanol (~750 g/l) TS.
Category. Diagnostic agent in ophthalmology.
Storage. Fluorescein sodium should be kept in a well-closed container, protected from light.
Additional information. Fluorescein sodium is hygroscopic.
Requirements
Definition. Fluorescein sodium contains not less than 98.0% and not more than 100.5% of C20H10Na2O5, calculated with reference to the dried substance.
Identity tests
A. A solution in water is strongly fluorescent, even in extreme dilution; the fluorescence disappears when the solution is made acid and reappears when it is made alkaline.
B. Ignite 20 mg and dissolve the residue in acetic acid (~60 g/l) TS. The solution yields reaction B described under 2.1 General identification tests as characteristic of sodium.
C. Dissolve 1 mg in 2 mL of water, place 0.05 mL of this solution on a piece of filter-paper; a yellow spot is produced. Expose the moist paper to the vapour of bromine R for 1 minute and then to the vapour of ammonia (~260 g/l) TS; the yellow colour of the spot changes to deep pink.
Chlorides. Dissolve 0.07 g in a mixture of 2 mL of nitric acid (~130 g/l) TS and 20 mL of water, and proceed as described under 2.2.1 Limit test for chlorides; the chloride content is not more than 3.5 mg/g.
Sulfates. Dissolve 0.05 g in 20 mL of water and proceed as described under 2.2.2 Limit test for sulfates; the sulfate content is not more than 10 mg/g.
Zinc. Dissolve 0.10 g in 10 mL of water, add 2 mL of hydrochloric acid (~420 g/l) TS, filter, and add 0.1 mL of potassium ferrocyanide (45 g/l) TS; no turbidity or precipitate is produced immediately.
Chloroform-soluble matter. Dissolve 0.20 g in 10 mL of sodium hydroxide (0.1 mol/l) VS and extract with 10 mL of chloroform R. Allow to separate, dry the chloroform layer over anhydrous sodium sulfate R, and filter. Measure the absorbance of the filtrate in a 1-cm cell at a maximum of 480 nm against a solvent cell containing chloroform R; the absorbance does not exceed 0.10.
Ethanol-insoluble matter. Boil 0.2 g with 20 mL of ethanol (~750 g/l) TS for 1 minute, filter through a sintered glass filter, wash the filter with ethanol (~750 g/l) TS until the filtrate is almost colourless, dry the filter at 105°C for 1 hour and weigh; the residue is not more than 2.0 mg.
Loss on drying. Dry to constant weight at 105°C; it loses not more than 100 mg/g.
pH value. pH of a 20 mg/mL solution in carbon-dioxide-free water R, 7.0-9.0.
Dimethylformamide. Carry out the test as described under 1.14.1 Chromatography, Gas chromatography. Prepare an internal standard consisting of a mixture of 20 μl of dimethylacetamide R in 100 mL of water. Inject the following 3 solutions: (1) a mixture of 2 μl of dimethylformamide R in 10 mL of water and containing 10 mL of the solution of the internal standard; (2) for the determination of the retention time of the substance being examined, dissolve 1.0 g of the test substance in 10 mL of water, add with stirring 10 mL of hydrochloric acid (0.5 mol/l) VS, allow to stand for 15 minutes, centrifuge, and then dissolve 0.10 g of trisodium orthophosphate R in 5 mL of the supernatant liquid; (3) dissolve 1.0 g of the test substance in 10 mL of the solution of the internal standard, add with stirring 10 mL of hydrochloric acid (0.5 mol/l) VS, allow to stand for 15 minutes, centrifuge, and then dissolve 0.10 g of trisodium orthophosphate R in 5 mL of the supernatant liquid.
For the procedure, use a glass column 1.5 m long and 4 mm in internal diameter packed with an adequate quantity of an adsorbent composed of 1 g of macrogol 1000 R supported on 9 g of acid-washed, silanized diatomaceous support R and maintained at 120°C. Use nitrogen R as the carrier gas and a flame ionization detector.
In the chromatogram obtained with solution 3, the ratio of the area of any peak due to dimethylformamide to the area of the peak due to the internal standard is not greater than the corresponding ratio for the chromatogram obtained with solution 1.
Resorcinol. Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography, using silica gel R1 as the coating substance (a precoated plate from a commercial source is suitable) and a mixture of 6 volumes of hexane R and 4 volumes of ethyl acetate R as the mobile phase. Apply separately to the plate 5 μl of each of the 2 following solutions: (A) dissolve 1.0 g of the test substance in 10 mL of water, add slowly with constant stirring 10 mL of hydrochloric acid (0.5 mol/l) VS, allow to stand for 15 minutes, centrifuge, and use the supernatant liquid; (B) dissolve 2.5 mg of resorcinol R in 10 mL of water. After removing the plate from the chromatographic chamber, allow it to dry in air, and expose it to the vapour of iodine R for 30 minutes. Examine the chromatogram in daylight. The spot obtained with solution B is more intense than any corresponding spot obtained with solution A.
Related substances. Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography, using silica gel R1 as the coating substance (a precoated plate from a commercial source is suitable) and a mixture of 8 volumes of chloroform R and 2 volumes of methanol R as the mobile phase. Apply separately to the plate 5 μl of each of 2 solutions in hydrochloric acid/methanol (0.1 mol/l) VS containing (A) 10 mg of the test substance per mL and (B) 20 μg of the test substance per mL. After removing the plate from the chromatographic chamber, allow it to dry in air, and expose the plate to the vapour of iodine R for 30 minutes. Examine the chromatogram in daylight. Any spot obtained with solution A, other than the principal spot, is not more intense than that obtained with solution B.
Assay. Dissolve about 0.5 g, accurately weighed, in 20 mL of water, add 5 mL of hydrochloric acid (~70 g/l) TS, and extract with 4 volumes, each of 20 mL, of a solvent mixture composed of equal volumes of 2-butanol R and chloroform R. Separate and combine the extracts, wash with 10 mL of water, extract the washings with 5 mL of the above solvent mixture, and add to the combined extracts. Evaporate the mixed extracts to dryness on a water-bath in a current of air, dissolve the residue in 10 mL of ethanol (~750 g/l) TS, evaporate to dryness on a water-bath and dry to constant weight at 105°C. Each g of residue is equivalent to 1.132 g of C20H10Na2O5.
Additional requirement for Fluorescein sodium for sterile use
Complies with 3.2 Test for sterility.