Monographs: Pharmaceutical substances: Furosemide (Furosemidum)
Molecular formula. C12H11ClN2O5S
Relative molecular mass. 330.8
Graphic formula.
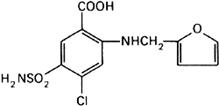
Chemical name. 4-Chloro-N-furfuryl-5-sulfamoylanthranilic acid; 5-(aminosulfonyl)-4-chloro-2-[(2-furanylmethyl)amino]benzoic acid; CAS Reg. No. 54-31-9.
Description. A white or almost white, crystalline powder; odourless.
Solubility. Practically insoluble in water; soluble in 75 parts of ethanol (~750 g/l) TS; slightly soluble in ether R.
Category. Diuretic.
Storage. Furosemide should be kept in a well-closed container, protected from light.
Requirements
Definition. Furosemide contains not less than 98.0% and not more than 101.0% of C12H11ClN2O5S, calculated with reference to the dried substance.
Identity tests
• Either test A alone or tests B and C may be applied.
A. Carry out the examination as described under 1.7 Spectrophotometry in the infrared region. The infrared absorption spectrum is concordant with the spectrum obtained from furosemide RS or with the reference spectrum of furosemide.
B. Dissolve 5 mg in 10 mL of methanol R. Transfer 1 mL of this solution to a flask, add 10 mL of hydrochloric acid (~70 g/l) TS and heat under a reflux condenser for 15 minutes. Cool and add 15 mL of sodium hydroxide (1 mol/l) VS and 5 mL of sodium nitrite (1 g/l) TS. Allow to stand for 3 minutes, then add 2 mL of ammonium sulfamate (25 g/l) TS and mix. Add 1 mL of N-(1-naphthyl)ethylenediamine hydrochloride (5 g/l) TS; a red-violet colour is produced.
C. Dissolve 25 mg in 2.5 mL of ethanol (~750 g/l) TS and add, drop by drop, about 2 mL of 4-dimethylaminobenzaldehyde TS1; a transient green colour is produced, which becomes deep red.
Heavy metals. Use 1.0 g for the preparation of the test solution as described under 2.2.3 Limit test for heavy metals. Procedure 3; determine the heavy metals content according to Method A; not more than 20 μg/g.
Sulfated ash. Not more than 1.0 mg/g.
Loss on drying. Dry to constant weight at 105°C; it loses not more than 5.0 mg/g.
4-Chloro-5-sulfamoylanthranilic acid. Dissolve 0.1 g in 25 mL of methanol R. To 1 mL add 3 mL of dimethylformamide R, 12 mL of water, and 1 mL of hydrochloric acid (1 mol/l) VS. Cool, add 0.5 mL of sodium nitrite (10 g/l) TS, shake, and allow to stand for 5 minutes. Add 1 mL of ammonium sulfamate (25 g/l) TS, shake, and allow to stand for 3 minutes. Add 1 mL of N-(1-naphthyl)ethylenediamine hydrochloride (5 g/l) TS and sufficient water to produce 25 mL. Measure the absorbance of a 1-cm layer of the resulting solution at a maximum of about 530 nm, using as a blank a solution prepared by treating a mixture of 1 mL of methanol R and 3 mL of dimethylformamide R in a similar manner; the absorbance is not greater than 0.12 (0.3% of free primary aromatic amines expressed as 4-chloro-5-sulfamoylanthranilic acid) (preferably use 2-cm cells for the measurement and calculate the absorbance of a 1-cm layer).
Related substances. Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography, using silica gel R1 as the coating substance and a mixture of 1 volume of toluene R, 1 volume of xylene R, 3 volumes of dioxan R, 3 volumes of 2-propanol R and 2 volumes of ammonia (~260 g/l) TS as the mobile phase. Apply separately to the plate 5 μl of each of 2 solutions in acetone R containing (A) 20 mg of the test substance per mL and (B) 0.30 mg of the test substance per mL. After removing the plate from the chromatographic chamber, allow it to dry in air, and examine the chromatogram in ultraviolet light (365 nm). Any spot obtained with solution A, other than the principal spot, is not more intense than that obtained with solution B.
Assay. Dissolve about 0.3 g, accurately weighed, in 40 mL of dimethylformamide R, add 3 drops of bromothymol blue/dimethylformamide TS, and titrate with sodium hydroxide (0.1 mol/l) VS to a blue end-point. Repeat the operation without the substance being examined and make any necessary corrections. Each mL of sodium hydroxide (0.1 mol/l) VS is equivalent to 33.08 mg of C12H11ClN2O5S.
Additional requirements for Furosemide for parenteral use
Complies with the monograph for "Parenteral preparations".
Bacterial endotoxins. Carry out the test as described under 3.4 Test for bacterial endotoxins; contains not more than 3.6 IU of endotoxin RS per mg.