Monographs: Pharmaceutical substances: Ganciclovir (Ganciclovirum)
Molecular formula. C9H13N5O4
Relative molecular mass. 255.2
Graphic formula
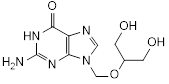
Chemical names. 2-amino-9-{[(1,3-dihydroxypropan-2-yl)oxy]methyl}-1,9-dihydro-6H-purin-6-one (IUPAC); 2-amino-1,9-dihydro-9-[[2-hydroxy-1-(hydroxymethyl)ethoxy]methyl]-6H-purin-6-one (CAS); CAS Reg. No. 82410-32-0.
Description. White or almost white, crystalline powder.
Solubility. Slightly soluble in water R or glacial acetic acid R, very slightly soluble in dehydrated ethanol R, practically insoluble in methanol R and dichloromethane R. It dissolves in dilute solutions of mineral acids and alkali hydroxides.
Category. Antiviral (Purine nucleoside analogue).
Storage. Preserve in well-closed containers. Protect from light and moisture.
Additional information. Ganciclovir is hygroscopic and may exhibit polymorphism. Caution: Ganciclovir is a potent cytotoxic agent and suspected carcinogen. It must be handled with care, avoiding contact with the skin and inhalation of airborne particles.
Requirements
Definition. Ganciclovir contains not less than 99.0% and not more than 101.0% of C9H13N5O4, calculated with reference to the anhydrous substance.
Identity tests
Either test A alone, or tests B and D or tests C and D may be applied.
A. Carry out the test as described under 1.7 Spectrophotometry in the infrared region. The infrared absorption spectrum is concordant with the spectrum obtained from ganciclovir RS or with the reference spectrum of ganciclovir.
If the spectra thus obtained are not concordant, repeat the test using the residues obtained by separately dissolving the test substance and ganciclovir RS in a small amount of hot water R (80 °C), allowing to cool in an ice-bath, filtering and drying the precipitate at 105 °C for 3 hours. The infrared absorption spectrum is concordant with the spectrum obtained from ganciclovir RS.
B. Carry out test B.1 or, where UV detection is not available, test B.2
B.1 Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography using silica gel R6 as the coating substance and a mixture of 4 volumes of ammonia (260 g/L) TS, 40 volumes of methanol R and 60 volumes of dichloromethane R as the mobile phase. Apply separately to the plate 5 μL of each of the following three solutions. For solution (A) dissolve 10 mg of the test substance in 2 mL of sodium hydroxide (~0.8 g/L) TS and dilute to 10 mL with methanol R. For solution (B) dissolve 10 mg of ganciclovir RS in 2 mL of sodium hydroxide (~0.8 g/L) TS and dilute to 10 mL with methanol R. For solution (C) dissolve 10 mg of ganciclovir RS and 10 mg of aciclovir R in 2 mL of sodium hydroxide (~0.8 g/L) TS and dilute to 10 mL with methanol R. After removing the plate from the chromatographic chamber allow it to dry exhaustively in air and examine the chromatogram under ultraviolet light (254 nm). The test is not valid unless the chromatogram obtained with solution (C) shows two clearly separated spots.
The principal spot in the chromatogram obtained with solution (A) corresponds in position, appearance and intensity with the spot due to ganciclovir in the chromatogram obtained with solution (B).
B.2 Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography using the conditions described above under test B.1 but using silica gel R5 as the coating substance. After removing the plate from the chromatographic chamber allow it to dry exhaustively in air or heat the plate for five minutes at 120 °C. Spray the plate with Dragendorff reagent TS and allow it to dry exhaustively in air.Then spray the plate with a mixture of sulfuric acid (~1760 g/L) TS and dehydrated ethanol R (1:1). Examine the chromatogram in daylight. The test is not valid unless the chromatogram obtained with solution (C) shows two clearly separated spots.
The principal spot in the chromatogram obtained with solution (A) corresponds in position, appearance and intensity with the spot due to ganciclovir in the chromatogram obtained with solution (B).
C. Carry out the test as described under 1.14.1 Chromatography, High-performance liquid chromatography using the conditions given under “Related substances”. The retention time of the principal peak in the chromatogram obtained with solution (1) corresponds to the retention time of the peak due to ganciclovir in the chromatogram obtained with solution (3).
D. Dissolve about 5 mg of the sample in 500 mL of water R. The absorption spectrum (1.6) of this solution, when observed between 200 nm and 300 nm, exhibits a minimum at about 222 nm and a maximum at about 252 nm with a shoulder at about 275 nm.
Clarity and colour of solution. Dissolve 1.25 g in sodium hydroxide (~40 g/L) TS and dilute to 25 mL. This solution is clear and not more intensely coloured than reference solution Y5, when compared as described under 1.11.2 Degree of coloration of liquids, Method II.
Heavy metals. Use 1.0 g for the preparation of the test solution as described under 2.2.3 Limit test for heavy metals, Procedure 3; determine the content of heavy metals according to Method A; not more than 10 μg/g.
Sulfated ash (2.3). Not more than 1.0 mg/g.
Water. Determine as described under 2.8 Determination of water by the Karl Fischer method, Method A, using 0.300 g of the substance and methanol as solvent. The substance to be examined has a limited solubility in methanol and will appear as a slurry. Replace the solvent after each titration. The water content is not more than 40 mg/g.
Related substances. Carry out the test as described under 1.14.4 High performance liquid chromatography using a stainless steel column (25 cm × 4.6 mm) packed with particles of silica gel, the surface of which has been modified with chemically-bonded strong acidic cation-exchange groups (3–10 μm).
Use the following mobile phase: Dilute 0.5 mL of trifluoroacetic acid R to 1000 mL with water R. Mix 500 volumes of this solution with 500 volumes of acetonitrile R.
Operate with a flow rate of 1.5 mL per minute. As a detector use an ultraviolet spectrophotometer set at a wavelength of 254 nm. Maintain the column at 40 °C.
Prepare the following solutions using mobile phase as a diluent. For solution (1) dissolve about 30 mg of the test substance using sonication and dilute to 50.0 mL. For solution (2) dilute 1 volume of solution (1) to 1000 volumes. For solution (3) dissolve 3.0 mg of ganciclovir RS using sonication and dilute to 5.0 mL. For solution (4) dissolve the content of a vial of ganciclovir for system suitability RS (containing the impurities A, B, C, D, E and F) in 1.0 mL of solution (3).
Inject alternately 20 μL each of solutions (1), (2), (3) and (4). Record the chromatograms for 2.5 times the retention time of ganciclovir (retention time about 14 minutes).
Use the chromatogram supplied with ganciclovir for system suitability RS and the chromatograms obtained with reference solution (3) and (4) to identify the peaks due to ganciclovir and the impurities A, B, C, D, E and F. The following peaks are eluted at the following relative retention with reference to the peak of ganciclovir: impurity A about 0.6; impurity B about 0.67; impurity C about 0.71; impurity D about 0.8; impurity E about 0.9; impurity F about 2.0.
The test is not valid unless in the chromatogram obtained with solution (4) the peak-to-valley ratio (Hp/Hv) is at least 5, where Hp is the height above the baseline of the peak due to impurity E and Hv is the height above the baseline of the lowest point of the curve separating this peak from the peak due to ganciclovir.
In the chromatogram obtained with solution (1):
- the area of any peak corresponding to impurity A, C, D or E is not greater than 1.5 times the area of the peak due to ganciclovir in the chromatogram obtained with solution (2) (0.15%);
- the area of any peak corresponding to impurity B, when multiplied by a correction factor of 1.3, is not greater than twice the area of the peak due to ganciclovir in the chromatogram obtained with solution (2) (0.2%);
- the area of any peak corresponding to impurity F, when multiplied by a correction factor of 0.7, is not greater than 4 times the area of the peak due to ganciclovir in the chromatogram obtained with solution (2) (0.4%);
- the area of any other impurity peak is not greater than 0.5 times the area of the peak due to ganciclovir in the chromatogram obtained with solution (2) (0.05%);
- the sum of the corrected areas of the peaks corresponding to impurity B and impurity F and the areas of all other impurity peaks is not greater than 6 times the area of the peak due to ganciclovir in the chromatogram obtained with solution (2) (0.6%). Disregard any peak with an area less than 0.3 times the area of the peak due to ganciclovir obtained with solution (2) (0.03%).
Assay. Dissolve 0.200 g in 10 mL of anhydrous formic acid R and dilute to 60 mL with anhydrous glacial acetic acid R. Titrate with perchloric acid (0.1 mol/L) VS, determining the end-point potentiometrically as described under 2.6 Non-aqueous titrations. Carry out a blank titration. Each mL of perchloric acid (0.1 mol/L) VS is equivalent to 25.52 mg of ganciclovir (C9H13N5O4).
Additional requirements for Ganciclovir for parenteral use
Complies with the monograph for Parenteral preparations.
Bacterial endotoxins. If intended for use in the manufacture of a parenteral dosage form without a further appropriate procedure for the removal of bacterial endotoxins, carry out the test as described under 3.4 Test for bacterial endotoxins; contains not more than 0.84 IU of endotoxin RS per mg of ganciclovir.
Impurities
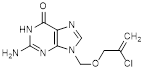
A. 2-amino-9-{[(2-chloroprop-2-en-1-yl)oxy]methyl}-1,9-dihydro-6H-purin-6-one (synthesis-related impurity),
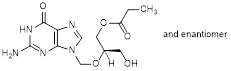
B. (2RS)-[(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methoxy]-3-hydroxypropyl propanoate (synthesis-related impurity),
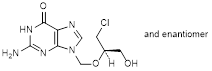
C. 2-amino-9-({[(2RS)-1-chloro-3-hydroxypropan-2-yl]oxy}methyl)-1,9-dihydro-6H-purin-6-one (synthesis-related impurity),
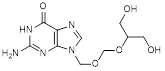
D. 2-amino-9-({[(1,3-dihydroxypropan-2-yl)oxy]methoxy}methyl)-1,9-dihydro-6H- purin-6-one (synthesis-related impurity),
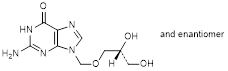
E. 2-amino-9-{[(2RS)-2,3-dihydroxypropoxy]methyl}-1,9-dihydro-6H-purin-6-one (isoganciclovir) (synthesis-related impurity) ,
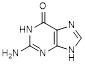
F. 2-amino-1,9-dihydro-6H-purin-6-one (guanine) (synthesis-related impurity, degradation product),
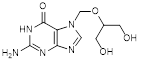
H. 2-amino-7-{[(1,3-dihydroxypropan-2-yl)oxy]methyl}-1,9-dihydro-6H-purin-6-one (synthesis-related impurity),
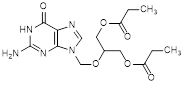
I. 2-[(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methoxy]propane-1,3-diyl bispropanoate (synthesis-related impurity),
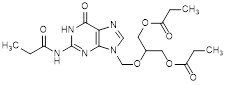
J. 2-[(6-oxo-2-propanamido-1,6-dihydro-9H-purin-9-yl)methoxy]propane-1,3-diyl bispropanoate (synthesis-related impurity).