Monographs: Pharmaceutical substances: Glycerol (Glycerolum)
Graphic formula.
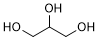
Molecular formula. C3H8O3
Relative molecular mass. 92.09
Chemical name. Glycerol; Propane-1,2,3-triol (IUPAC); 1,2,3-Propanetriol (CAS).
CAS Registry Number. 56-81-5.
Other name. Glycerin.
Description. A clear, colourless or almost colourless, syrupy liquid; odourless.
Miscibility. Miscible with water and ethanol (~750 g/l) TS; slightly miscible with acetone R; practically immiscible with ether R.
Category. Solvent; humectant.
Storage. Glycerol should be kept in a tightly closed container.
Additional information. Glycerol is hygroscopic. The substance is at risk for ethylene glycol (EG) or diethylene glycol (DEG) contamination and must therefore be tested for these contaminants before it is used in the production of medicines. The International Pharmacopoeia provides analytical test procedures for the detection of EG and DEG in liquid preparations for oral use in the Supplementary Information section, which are transferable to pharmaceutical substances. Pharmaceutical manufacturers are expected to use the gas chromatography method to test for DEG/EG contamination.
Requirements
Glycerol contains not less than 95.0% and not more than the equivalent of 101.0% of C3H8O3, calculated with reference to the anhydrous substance.
Identity tests
A. Impregnate a piece of filter-paper with alkaline potassio-mercuric iodide TS; place it over a test-tube containing 1 mL of Glycerol with 2 g of potassium hydrogen sulfate R and heat; the paper turns black.
B. Mix 2 g with 10 mL of water and add 1 drop of phenolphthalein/ethanol TS; the solution remains colourless. Add 1 drop of methyl red/ethanol TS; the colour changes to yellow.
C. Mix 1 mL with 0.5 mL of nitric acid (~1000 g/l) TS and superimpose 0.5 mL of potassium dichromate (100 g/l) TS; a blue ring is produced at the interface of the two liquids. Allow to stand for 10 minutes; the blue colour does not diffuse into the lower layer.
Refractive index. 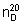 = 1.470 - 1.475
= 1.470 - 1.475
Relative density. 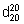 = 1.258 - 1.263.
= 1.258 - 1.263.
Heavy metals. Use 1.0 g for the preparation of the test solution as described under 2.2.3 Limit test for heavy metals, Procedure 1; determine the heavy metals content according to Method A; not more than 5 μg/g.
Chlorides. Use 5 g in a mixture of 2 mL of nitric acid (~130 g/l) TS and 20 mL of water, and proceed as described under 2.2.1 Limit test for chlorides, using 1.0 mL of hydrochloric acid ClTS; the chloride content is not more than 10 μg/g.
Sulfates. Use 24 g and proceed as described under 2.2.2 Limit test for sulfates; the sulfate content is not more than 20 μg/g.
Clarity and colour of solution. Mix 25 g with sufficient water to produce 50 mL; the solution is clear. Dilute 10 mL of this solution to 25 mL with water; the solution is colourless.
Chlorinated compounds. Place about 5 g, accurately weighed, in a dry round-bottomed 100-mL flask, add 15 mL of morpholine R, and connect to a suitable reflux condenser. Reflux gently for 3 hours. Rinse the condenser with 10 mL of water, adding the washings back into the flask, and cautiously acidify with nitric acid (~1000 g/l) TS. Transfer the solution to a suitable comparison tube, add 0.5 mL of silver nitrate (0.1 mol/l) VS, dilute with water to exactly 50 mL, and mix thoroughly; the turbidity is not more pronounced than that of a solution prepared similarly but without refluxing and to which 0.2 mL of hydrochloric acid (0.02 mol/l) VS has been added (30 μg of Cl/g).
Acidity. Dilute 25 g to 50 mL with carbon-dioxide-free water R and add 0.5 mL of phenolphthalein/ethanol TS; the solution remains colourless. Titrate with carbonate-free sodium hydroxide (0.1 mol/l) VS; not more than 0.2 mL is required to obtain a pink colour. (Keep the solution for "Fatty acids and esters".)
Fatty acids and esters. To the above solution, add 5 mL of carbonate-free sodium hydroxide (0.5 mol/l) VS, boil the mixture for 5 minutes, cool, add phenolphthalein/ethanol TS, and titrate with hydrochloric acid (0.5 mol/l) VS. Repeat the procedure without the Glycerol being examined and make any necessary corrections. Not more than 1.0 mL of carbonate-free sodium hydroxide (0.5 mol/l) VS is consumed.
Aldehydes and reducing substances. Transfer 5 mL of Glycerol to a glass-stoppered flask, mix with 10 mL of water and 1 mL of fuchsin/sulfurous acid TS. Allow the mixture to stand in the dark for 1 hour; the colour of the solution is not more intense than that of a solution of potassium permanganate (0.0002 mol/l) VS.
Sulfated ash. Not more than 0.1 mg/g.
Water. Determine as described under 2.8 Determination of water by the Karl Fischer method, Method A, using 1.5 g of Glycerol; the water content is not more than 20 mg/g.
Assay. Transfer about 0.4 g, accurately weighed, to a 600-mL beaker, dilute with 50 mL of water, add bromothymol blue/ethanol TS, and acidify with sulfuric acid (0.1 mol/l) VS to a green or greenish yellow colour. Neutralize with sodium hydroxide (0.05 mol/l) VS to a definite blue end-point, showing no green tinge. Prepare a reagent blank containing 50 mL of water, and neutralize in the same manner. Pipette 50 mL of sodium metaperiodate TS into each beaker, swirl gently to mix, cover with a watch-glass, and allow to stand for 30 minutes at room temperature (not exceeding 35 °C). Dilute each solution with water to about 300 mL and, using a pH-meter, titrate with sodium hydroxide (0.1 mol/l) VS to pH 8.1 ± 0.1 for Glycerol and pH 6.5 ± 0.1 for the blank. Make any necessary corrections for the blank.
Each mL of sodium hydroxide (0.1 mol/l) VS is equivalent to 9.210 mg of C3H8O3.