Monographs: Pharmaceutical substances: Haloperidol (Haloperidolum)
Molecular formula. C21H23ClFNO2
Relative molecular mass. 375.9
Graphic formula.
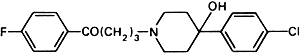
Chemical name. 4-[4-(p-Chlorophenyl)-4-hydroxypiperidino]-4'-fluorobutyrophenone; 4-[4-(4-chlorophenyl)-4-hydroxy-1-piperidinyl]-1-(4-fluorophenyl)-1-butanone; CAS Reg. No. 52-86-8.
Description. A white to faintly yellowish, amorphous or microcrystalline powder; odourless.
Solubility. Practically insoluble in water; soluble in 50 parts of ethanol (~750 g/l) TS and in 200 parts of ether R.
Category. Neuroleptic.
Storage. Haloperidol should be kept in a well-closed container, protected from light.
Requirements
Definition. Haloperidol contains not less than 98.0% and not more than 101.0% of C21H23ClFNO2, calculated with reference to the dried substance.
Identity tests
• Either tests A and C or tests B and C may be applied.
A. Carry out the examination as described under 1.7 Spectrophotometry in the infrared region. The infrared absorption spectrum is concordant with the spectrum obtained from haloperidol RS or with the reference spectrum of haloperidol.
B. The absorption spectrum of a 15 μg/mL solution in a mixture of 1 volume of hydrochloric acid (1 mol/l) VS and 99 volumes of methanol R, when observed between 230 nm and 350 nm, exhibits a maximum at about 245 nm; the absorbance of a 1-cm layer at this wavelength is between 0.49 and 0.53 (preferably use 2-cm cells for the measurement and calculate the absorbance of a 1-cm layer).
C. Carry out the combustion as described under 2.4 Oxygen flask method, using 20 mg of the test substance and a mixture of 3 mL of sodium hydroxide (~80 g/l) TS and 2 mL of water as the absorbing liquid. When the process is complete, dilute to 10 mL with water; the resulting solution complies with the following tests:
(a) Add 0.1 mL to a mixture of 0.1 mL of a freshly prepared sodium alizarinsulfonate (1 g/l) TS and 0.1 mL of zirconyl nitrate TS; the red colour of the solution changes to clear yellow.
(b) Acidify 5 mL with sulfuric acid (~100 g/l) TS and boil gently for 2 minutes; the solution yields reaction A described under 2.1 General identification tests as characteristic of chlorides.
Melting range. 147-152°C.
Sulfated ash. Not more than 1.0 mg/g.
Loss on drying. Dry to constant weight at 60°C under reduced pressure (not exceeding 0.6 kPa or about 5 mm of mercury); it loses not more than 5.0 mg/g.
Related substances. Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography, using silica gel R5 as the coating substance (a precoated plate is preferable) and a mixture of 80 volumes of chloroform R, 10 volumes of glacial acetic acid R and 10 volumes of methanol R as the mobile phase. Apply separately to the plate 10 μl of each of 3 solutions in chloroform R containing (A) 10 mg of the test substance per mL, (B) 0.050 mg of the test substance per mL, and (C) 0.10 mg of the test substance per mL. After removing the plate from the chromatographic chamber, allow it to dry in air, spray with potassium iodobismuthate TS2, and examine the chromatogram in daylight. Any spot obtained with solution A, other than the principal spot, is not more intense than that obtained with solution B. Not more than one of any such spots is more intense than the spot obtained with solution C.
Assay. Dissolve about 0.35 g, accurately weighed, in 30 mL of glacial acetic acid R1 and titrate with perchloric acid (0.1 mol/l) VS, determining the end-point potentiometrically as described under 2.6 Non-aqueous titration, Method A. Each mL of perchloric acid (0.1 mol/l) VS is equivalent to 37.59 mg of C21H23ClFNO2.
Additional requirements for Haloperidol for parenteral use
Complies with the monograph for "Parenteral preparations".
Bacterial endotoxins. Carry out the test as described under 3.4 Test for bacterial endotoxins; contains not more than 71.4 IU of endotoxin RS per mg.