Monographs: Pharmaceutical substances: Halothane (Halothanum)
Molecular formula. C2HBrClF3
Relative molecular mass. 197.4
Graphic formula.
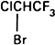
Chemical name. 2-Bromo-2-chloro-1,1,1-trifluoroethane; CAS Reg. No. 151-67-7.
Description. A colourless, mobile, heavy liquid; odour, characteristic, resembling that of chloroform.
Miscibility. Miscible with 400 parts of water; miscible with ethanol (~750 g/l) TS, ether R, and trichloroethylene R.
Category. General anaesthetic.
Storage. Halothane should be kept in a well-closed container, protected from light, and stored at a temperature not exceeding 25°C.
Additional information. Halothane is a noninflammable liquid. Halothane contains not less than 0.08 mg/g and not more than 0.12 mg/g of thymol, as a stabilizer.
Requirements
Identity tests
To a test-tube, transfer 2 mL of tert.-butanol R, 0.1 mL of the test liquid, 1 mL of copper edetate TS, 0.5 mL of ammonia (~260 g/l) TS and 2 mL of hydrogen peroxide (~60 g/l) TS; this constitutes solution 1. Similarly, prepare a blank without the test liquid; this constitutes solution 2. Place both tubes in a water-bath at 50°C for 15 minutes, cool and add 0.3 mL of glacial acetic acid R.
A. To 1 mL of each of solutions 1 and 2 add 0.5 mL of a mixture of equal volumes of sodium alizarinsulfonate (1 g/l) TS and zirconyl nitrate TS; a red colour is produced in solution 2 and a yellow colour in solution 1.
B. To 1 mL of each of solutions 1 and 2 add 1 mL of a mixture of 1.02 g of potassium hydrogen phthalate R dissolved in 30 mL of sodium hydroxide (0.1 mol/l) VS and diluted to 100 mL with water (= buffer pH 5.2). Then add (a) 1 mL of phenol red/ethanol TS that has been previously diluted with an equal volume of water and (b) 0.1 mL of tosylchloramide sodium (15 g/l) TS; a yellow colour is produced in solution 2 and a bluish red colour in solution 1.
C. To 2 mL of each of solutions 1 and 2 add 0.5 mL of sulfuric acid (~570 g/l) TS, 0.5 mL of acetone R and 0.2 mL of potassium bromate (50 g/l) TS. Shake, then place in a water-bath at 50°C for 2 minutes. Cool, add 0.5 mL of a mixture of equal volumes of nitric acid (~1000 g/l) TS and water, and 0.1 mL of silver nitrate (40 g/l) TS; solution 2 remains clear and an opalescence is produced in solution 1, which changes to a white precipitate after a few minutes.
Mass density, ρ20 = 1.865-1.875 g/mL.
Free halides. Shake 10 mL with 20 mL of carbon-dioxide-free water R for 3 minutes. To 5 mL of the aqueous layer add 5 mL of water, 1 drop of nitric acid (~1000 g/l) TS, and 0.2 mL of silver nitrate (40 g/l) TS; no opalescence is produced. (Keep the remaining aqueous layer for the test of free halogens).
Free halogens. To 10 mL of the aqueous layer obtained from the test for free halides add 1 mL of potassium iodide/starch TS; no blue colour is produced.
Acidity or alkalinity. Shake 20 mL with 20 mL of carbon-dioxide-free water R for 3 minutes. To the aqueous layer add a few drops of bromocresol purple/ethanol TS; not more than 0.1 mL of sodium hydroxide (0.01 mol/l) VS or 0.6 mL of hydrochloric acid (0.01 mol/l) VS is required to obtain the midpoint of the indicator (grey).
Thymol. Use 3 dry 25-mL stoppered cylinders and place in the first one 0.5 mL of the test liquid, in the second one 0.5 mL of thymol TS2, and in the third one 0.5 mL of thymol TS3. To each of the 3 cylinders add 5 mL of carbon tetrachloride R and 5.0 mL of titanium dioxide/sulfuric acid TS. Shake the cylinders vigorously for 30 seconds and allow to stand until the layers have separated. When viewed transversally the yellowish-brown colour of the lower layer obtained in the cylinder containing the test liquid is intermediate in intensity between the colours of the corresponding layers in the other 2 cylinders (0.08-0.12 mg/g of thymol).
Related substances. Carry out the test as described under 1.14.1 Chromatography, Gas chromatography, using 3 solutions (1) trichlorotrifluoroethane TS serving as an internal standard, (2) the test liquid, and (3) the test liquid containing 0.05 μl of trichlorotrifluoroethane R per mL.
For the procedure use a glass column 2.75 m long and 5.0 mm in internal diameter, the first 1.8 m of which are packed with an adequate quantity of an adsorbent composed of 30 g of macrogol 400 R supported on 70 g of pink firebrick R, and the remainder with an adequate quantity of an adsorbent composed of 30 g of dinonyl phthalate R supported on 70 g of pink firebrick R. Maintain the column at 50°C, use nitrogen R as the carrier gas and a flame ionization detector.
In the chromatogram obtained with solution 3, the peak area due to the trichlorotrifluoroethane is greater than the total area of any other peaks except that due to the halothane; in calculating the peak area due to the trichlorotrifluoroethane, allowance may be necessary for any impurities having the same retention time as revealed in the chromotogram obtained from solution 2.