Monographs: Pharmaceutical substances: Homatropine hydrobromide (Homatropini hydrobromidum)
Molecular formula. C16H21NO3,HBr
Relative molecular mass. 356.3
Graphic formula.
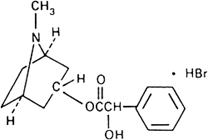
Chemical name. 1αH,5αH-Tropan-3α-ol mandelate (ester) hydrobromide; (±)-endo-8-methyl-8-azabicyclo[3.2.1]oct-3-yl α-hydroxybenzeneacetate hydrobromide; CAS Reg. No. 51-56-9.
Description. Colourless crystals or a white, crystalline powder; odourless.
Solubility. Freely soluble in water; sparingly soluble in ethanol (~750 g/l) TS; practically insoluble in ether R.
Category. Mydriatic.
Storage. Homatropine hydrobromide should be kept in a tightly closed container, protected from light.
Requirements
Definition. Homatropine hydrobromide contains not less than 98.5% and not more than 101.0% of C16H21NO3,HBr, calculated with reference to the dried substance.
Identity tests
A. Dissolve 10 mg in 1 mL of water, add ammonia (~100 g/l) TS to render the solution slightly alkaline, and shake with 5 mL of chloroform R. Evaporate the chloroform layer to dryness on a water-bath and add 1.5 mL of mercuric chloride/ethanol TS to the residue; a yellow colour is produced, which turns red on heating.
B. A 20 mg/mL solution yields reaction A described under 2.1 General identification tests as characteristic of bromides.
C. Melting temperature, about 215°C with decomposition.
Sulfated ash. Not more than 1.0 mg/g.
Loss on drying. Dry to constant weight at 105°C; it loses not more than 15 mg/g.
pH value. pH of a 20 mg/mL solution, 5.5-7.0.
Foreign alkaloids. Dissolve 10 mg in 2 mL of water and add 0.25 mL of tannic acid (50 g/l) TS; no precipitate is produced.
Related alkaloids. Dissolve 5 mg in 0.25 mL of fuming nitric acid R and evaporate to dryness on a water-bath. Allow to cool, add 0.1 mL of acetone R and 0.1 mL of a mixture of 1 volume of potassium hydroxide/ethanol (0.5 mol/l) VS and 4 volumes of aldehyde-free ethanol (~750 g/l) TS; no violet or reddish violet colour is produced.
Assay.
Dissolve 0.300 g in a mixture of 5.0 mL of hydrochloric acid (0.01 mol/L) VS and 50 mL of dehydrated ethanol R. Carry out a potentiometric titration using sodium hydroxide (0.1 mol/L) VS, as described under 2.6 Non-aqueous titration. Read the volume added between the two points of inflexion.
1 mL of sodium hydroxide (0.1 mol/L) VS is equivalent to 35.63 mg of C16H21NO3,HBr.