Monographs: Pharmaceutical substances: Hydrocortisone sodium succinate (Hydrocortisoni natrii succinas)
Molecular formula. C25H33NaO8
Relative molecular mass. 484.5
Graphic formula.
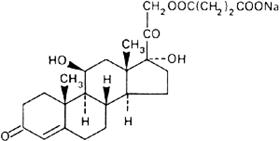
Chemical name. Cortisol 21-(sodium succinate); 21-(3-carboxy-1-oxopropoxy)-11β,17-dihydroxypregn-4-ene-3,20-dione monosodium salt; CAS Reg. No. 125-04-2.
Description. A white or almost white, crystalline powder or amorphous solid; odourless.
Solubility. Freely soluble in water; soluble in 34 parts of ethanol (~750 g/l) TS and in 200 parts of dehydrated ethanol R; practically insoluble in ether R.
Category. Adrenal hormone.
Storage. Hydrocortisone sodium succinate should be kept in a tightly closed container, protected from light.
Additional information. Hydrocortisone sodium succinate is hygroscopic. Even in the absence of light, it is gradually degraded on exposure to a humid atmosphere, the decomposition being faster at higher temperatures.
Requirements
Definition. Hydrocortisone sodium succinate contains not less than 97.0% and not more than 103.0% of C25H33NaO8, calculated with reference to the dried substance.
Identity tests
A. Carry out the examination as described under 1.7 Spectrophotometry in the infrared region. The infrared absorption spectrum is concordant with the spectrum obtained from hydrocortisone sodium succinate RS or with the reference spectrum of hydrocortisone sodium succinate.
B. Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography, using silica gel R1 as the coating substance and a freshly prepared mixture of 3 volumes of 1-butanol R, 1 volume of acetic anhydride R, and 1 volume of water as the mobile phase. Apply separately to the plate 2 μl of each of 2 solutions in methanol R containing (A) 2.5 mg of the test substance per mL and (B) 2.5 mg of hydrocortisone sodium succinate RS per mL. After removing the plate from the chromatographic chamber, allow it to dry in air until the solvents have evaporated, spray it with a mixture of 10 mL of sulfuric acid (~1760 g/l) TS and 90 mL of ethanol (~750 g/l) TS, heat it at 120°C for 10 minutes, allow it to cool, and examine the chromatogram in ultraviolet light (365 nm). The principal spot obtained with solution A corresponds in position, appearance, and intensity with that obtained with solution B.
C. When tested for sodium as described under 2.1 General identification tests, it yields the characteristic reactions. If reaction B is to be used, prepare a 20 mg/mL solution.
Specific optical rotation. Use a 10 mg/mL solution in ethanol (~750 g/l) TS and calculate with reference to the dried substance;  = +140° to +150°.
= +140° to +150°.
Loss on drying. Dry to constant weight at 105°C; it loses not more than 30 mg/g.
Related steroids. Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography, using silica gel R1 as the coating substance and a mixture of 77 volumes of dichloromethane R, 15 volumes of ether R, 8 volumes of methanol R, and 1.2 volumes of water as the mobile phase. Apply separately to the plate 1 μl of each of 2 solutions in a mixture of equal volumes of chloroform R and methanol R containing (A) 15 mg of the test substance per mL and (B) 0.15 mg of the test substance per mL. After removing the plate from the chromatographic chamber, allow it to dry in air until the solvents have evaporated, then heat it at 105°C for 10 minutes; allow it to cool, spray it with blue tetrazolium/sodium hydroxide TS, and examine the chromatogram in daylight. Any spot obtained with solution A, other than the principal spot, is not more intense than that obtained with solution B.
Sodium. Dissolve with gentle heating about 1 g, accurately weighed, in 75 mL of glacial acetic acid R. Add 20 mL of dioxan R, 0.15 mL of crystal violet/acetic acid TS, and titrate with perchloric acid (0.1 mol/l) VS. Each mL of perchloric acid (0.1 mol/l) VS is equivalent to 2.299 mg of Na; the content is not less than 46.0 mg and not more than 48.4 mg of Na per g, calculated with reference to the dried substance.
Assay. Dissolve about 20 mg, accurately weighed, in sufficient water to produce 100 mL; dilute 5 mL to 100 mL with water. Measure the absorbance of a 1-cm layer of the diluted solution at the maximum at about 248 nm. Calculate the amount of C25H33NaO8 in the substance being tested by comparison with hydrocortisone sodium succinate RS, similarly and concurrently examined. In an adequately calibrated spectrophotometer the absorbance of the reference solution should be 0.34 ± 0.02.