Monographs: Pharmaceutical substances: Hydroxypropylcellulose, low-substituted (Hydroxypropylcellulosum substitutum humile)
This monograph is based on the corresponding, internationally-harmonized text developed by the Pharmacopoeial Discussion Group (PDG). Editorial modifications have been made in order to be in line with the style used in The International Pharmacopoeia.
Chemical name. Cellulose 2-hydroxypropyl ether, low-substituted. CAS Reg. No. 9004-64-2.
Description. White or yellowish-white powder or granules.
Solubility. Practically insoluble in ethanol (~750 g/L) TS. Dissolves in a dilute solution of sodium hydroxide producing a viscous solution. Swells in water, in sodium carbonate (106 g/L) TS and in hydrochloric acid (~206 g/L) TS.
Category. Disintegrant; binder.
Storage. Low-substituted hydroxypropylcellulose should be kept in an airtight container.
Additional information. Low-substituted hydroxypropylcellulose is hygroscopic.
Requirements
Definition. Low-substituted hydroxypropylcellulose is a cellulose in which a low proportion of the hydroxyl groups have been replaced by 2-hydroxypropoxy groups. It contains not less than 5.0% and not more than 16.0% of hydroxypropoxy groups (–OCH2CHOHCH3), calculated with reference to the dried substance.
Identity tests
-
Carry out the test as described under 1.7 Spectrophotometry in the infrared region. The infrared absorption spectrum is concordant with the spectrum obtained from low-substituted hydroxypropylcellulose RS or with the reference spectrum of low-substituted hydroxypropylcellulose.
-
Shake 0.1 g of the test substance thoroughly with 10 mL of water R; it does not dissolve.
-
To the suspension obtained in test B, add 1 g of sodium hydroxide R and shake until it becomes homogeneous. Transfer 5 mL of the solution to a suitable container, add 10 mL of a mixture of 1 volume of methanol R and 4 volumes of acetone R, and shake; a white, flocculent precipitate is formed.
pH value (1.13). Evenly distribute 1.0 g of the test substance onto the surface of 100 mL of carbon-dioxide-free water R and stir using a magnetic stirrer. pH of the suspension, 5.0 to 7.5.
Loss on drying. Dry 1.000 g of the test substance at 105 °C for 1 hour; it loses not more than 50 mg/g.
Sulfated ash (2.3). Determine on 1.0 g of the test substance using Method B; not more than 8 mg/g.
Assay. Carry out the test as described under 1.14.1 Chromatography, Gas chromatography, using the internal standard method.
Use a fused-silica column (30 m × 0.53 mm) which is coated with polydimethylsiloxane R (3 μm). If necessary, also use a pre-column. Maintain the temperature of the column at 50 °C for 3 minutes. Increase the temperature at a rate of 10 °C per minute to 100 °C, and then at a rate of 37.5 °C per minute to 250 °C, and maintain it at this temperatures for 8 minutes. Maintain the temperature of the injection port and the detector at 250 °C and 280 °C, respectively. Use helium R as the carrier gas at an appropriate pressure with a flow rate of 4.3 mL per minute. Use a split ratio of 1:40. Use either a flame-ionization detector or a thermal conductivity detector.
Prepare an internal standard solution containing 30 mg/mL of octane R in o-xylene R.
Use the following apparatus in the preparation of solutions (1) and (2):
- Reaction vial. A 5 mL pressure-tight vial, 50 mm in height, 20 mm in external diameter and 13 mm in internal diameter at the mouth, equipped with a pressure-tight butyl rubber membrane stopper coated with polytetrafluoroethylene and secured with an aluminium crimped cap or another sealing system providing a sufficient air-tightness.
- Heater. A heating module with a square aluminium block having holes 20 mm in diameter and 32 mm in depth, so that the reaction vials fit; mixing of the contents of the vial is effected using a magnetic stirrer equipped in the heating module or using a reciprocal shaker that performs approximately 100 cycles/minute.
Note: Hydriodic acid and its reaction by-products are highly toxic. All operations in the preparation of solutions (1) and (2) should be conducted under a well-ventilated fume hood.
Prepare the solutions as follows: for solution (1), weigh 65.0 mg of the test substance, place in a reaction vial, add 0.06-0.10 g of adipic acid R, 2.0 mL of the internal standard solution and 2.0 mL of hydriodic acid R1, immediately cap and seal the vial, and weigh accurately. Mix the contents of the vial continuously for 60 minutes while heating the block so that the temperature of the contents is maintained at 128 °C to 132 °C. If a reciprocal shaker or magnetic stirrer cannot be used, shake the vial thoroughly by hand at 5-minute intervals during the initial 30 minutes of the heating time. Allow the vial to cool, and again weigh accurately. If the loss of mass is less than 26 mg and there is no evidence of a leak, use the upper layer of the mixture. For solution (2), place 0.06-0.10 g of adipic acid R, 2.0 mL of the internal standard solution and 2.0 mL of hydriodic acid R in another reaction vial, cap and seal the vial, and weigh accurately. Add 15-22 µL of isopropyl iodide R through the septum with a syringe and weigh accurately. Shake the reaction vial thoroughly and use the upper layer.
Inject separately 1-2 µL each of solutions (1) and (2) and record the chromatograms.
In the chromatogram obtained with solution (2), the peak for the analyte isopropyl iodide is eluted at a relative retention of about 0.8 with reference to octane (retention time about 8 minutes). The test is not valid unless the resolution between the peaks corresponding to isopropyl iodide and octane in the chromatogram obtained with solution (2) is at least 5.0. The test is also not valid if the maximum relative standard deviation for the ratio of the area of the peak due to isopropyl iodide to that due to octane in the chromatogram obtained with solution (2) determined on 6 injections is more than 2.0%.
Calculate the ratio (Q) of the area of the peak due to isopropyl iodide to the area of the peak due to the internal standard from the chromatogram obtained with solution (1), and the ratio (Q1) of the area of the peak due to isopropyl iodide to the area of the peak due to the internal standard from the chromatogram obtained with solution (2).
Calculate the percentage content of hydroxypropoxy groups (–OCH2 CHOHCH3), using the following expression:
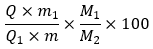
where
m1 = mass of isopropyl iodide in solution (2), in milligrams
m = mass of the sample (dried substance), in milligrams
M1 = molar mass of hydroxypropoxy group (75.1)
M2 = molar mass of isopropyl iodide (170.0)