Monographs: Pharmaceutical substances: Iohexol (Iohexolum)
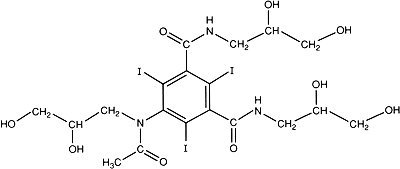
C19H26I3N3O9
Relative molecular mass. 821.1
Chemical name. N,N'-Bis(2,3-dihydroxypropyl)-5-[N-(2,3-dihydroxypropyl)-acetamido]-2,4,6-triiodoisophthalamide; 5-[acetyl(2,3-dihydroxypropyl)amino]-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide; CAS Reg. No. 66108-95-0.
Description. A white to greyish, amorphous powder; odourless.
Solubility. Very soluble in water and methanol R.
Category. Radiocontrast medium.
Storage. Iohexol should be kept in a well-closed container, protected from light.
Additional information. Melting range, 177-187 °C.
Requirements
Definition. Iohexol contains not less than 98.5% and not more than the equivalent of 101.5% of C19H26I3N3O9, calculated with reference to the anhydrous substance.
Identity tests
• Either test A alone or tests B, C, and D may be applied.
A. Carry out the examination as described under 1.7 Spectrophotometry in the infrared region. The infrared absorption spectrum is concordant with the spectrum obtained from iohexol RS or with the reference spectrum of iohexol.
B. The absorption spectrum of a 10μg/mL solution, when observed between 230nm and 350nm, exhibits a maximum at about 245 nm; the absorbance of a 1-cm layer at the maximum wavelength is about 0.36.
C. See the test described below under "Related substances". The principal spot obtained with solution A corresponds in position, appearance, and intensity with that obtained with solution B.
D. Heat 0.05 g in a suitable crucible; violet vapours are evolved.
Aluminium. Place in three separatory funnels 2 mL of a freshly prepared aluminium standard (10 μg Al/mL) TS, 10 mL of a solution containing 0.5 g of Iohexol per mL, and 10 mL of water to serve as a blank. To each funnel add 5 mL of ammonium chloride buffer, pH 10.5, TS and dilute to 25 mL with water. To every funnel add 5 mL of 8-hydroxyquinoline/chloroform TS and shake for 2 minutes. Allow the phases to separate and measure the absorbance of the extracts against the extracted blank solution at a maximum at about 395 nm; the aluminium content is not more than 4 μg/g.
Copper. Place in three separatory funnels 0.25 mL of a freshly prepared copper standard (10 μg/mL Cu) TS, 10 mL of a solution containing 0.5 g of Iohexol per mL, and 15 mL of water to serve as a blank. To each funnel add 1 mL of ammonium pyrrolidinedithiocarbamate (10 g/l) TS and 5 mL of acetate buffer, pH 4.5, TS and dilute to 25 mL with water. To each funnel add 5 mL of isobutyl methyl ketone R and shake for 2 minutes. Allow the phases to separate and measure the absorbance of the extracts against the extracted blank solution at a maximum at about 435 nm; the copper content is not more than 0.5 μg/g.
Halides. Dissolve 5 g in 20 mL of water and titrate with silver nitrate (0.001 mol/l) VS, determining the end-point potentiometrically, using silver/silver chloride electrodes. Repeat the procedure without the Iohexol being examined and make any necessary corrections. Each mL of silver nitrate (0.001 mol/l) VS is equivalent to 0.1269 mg of I; the content of halides, calculated as iodides, does not exceed 20 μg/g.
Colour of solution. Dissolve 6.47 g in sufficient water to produce 10 mL (apply a correction for any presence of water). The concentration of the solution is 64.7%, equivalent to 300 mg of 1 per mL. Filter through a Millipore filter 0.22 μm, and measure the absorbance in a 1-cm cell using water as a blank at about 400 nm, 420 nm, and 450 nm. The absorbance is not greater than 0.200, 0.050, and 0.025, respectively.
Water. Determine as described under 2.8 Determination of water by the Karl Fischer method, Method A, using 0.2 g; the water content is not more than 50 mg/g.
Related substances. Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography, using silica gel R6 as the coating substance and a mixture of 50 volumes of 1-butanol R, 11 volumes of acetic acid (~300 g/l) TS, and 25 volumes of water as the mobile phase. Apply separately to the plate 10 μl of each of four solutions in methanol R containing (A) 10 mg of Iohexol per mL, (B) 10 mg of iohexol RS per mL, (C) 20 mg of Iohexol per mL, and (D) 40μg of Iohexol per mL. After removing the plate from the chromatographic chamber, allow it to dry in air, and examine the chromatogram in ultraviolet light (254 nm).
Any spot obtained with solution C, other than the principal spot, is not more intense than that obtained with solution D.
Primary aromatic amines
• The solutions must be protected from light throughout the procedure.
To about 0.2 g, accurately weighed, add 15 mL of water, mix to dissolve, and allow to stand in an ice-bath for 5 minutes. Add 1.5 mL of hydrochloric acid (~250 g/l) TS and 2 mL of sodium nitrite (10 g/l) TS, mix, and return the flask to the ice-bath for exactly 4 minutes. Add 1 mL of sulfamic acid (50 g/l) TS and again place the flask in the ice-bath for exactly 1 minute. Remove the flask from the bath, add 0.5 mL of freshly prepared N-(1-naphthyl)ethylenediamine hydrochloride/propylene glycol TS, mix, and dilute to 25 mL with water. Within 20 minutes measure the absorbance of a 5-cm layer at the maximum at about 495 nm against a solvent cell containing a solution prepared by treating 15 mL of water in a similar manner; the absorbance does not exceed 0.21.
Assay. Carry out the combustion as described under 2.4 Oxygen flask method for iodine, using about 7.5 mg, accurately weighed. Titrate the liberated iodine with sodium thiosulfate (0.01 mol/l) VS.
Each mL of sodium thiosulfate (0.01 mL/l) VS is equivalent to 0.4562 mg of C19H26I3N3O9.