Monographs: Pharmaceutical substances: Kanamycin monosulfate (Kanamycini monosulfas)
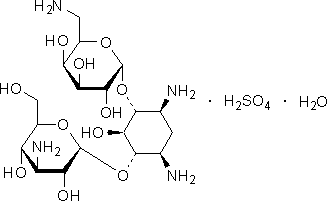
C18H36N4O11 . H2O4S . H2O
Relative molecular mass. 600.6
Chemical name. 4-O-(3-amino-3-deoxy-α-D-glucopyranosyl)-6-O-(6-amino-6-deoxy-α-D-glucopyranosyl)-2-deoxy-L-streptamine monosulfate monohydrate; 3-amino-3-deoxy-α-D-glucopyranosyl-(1→6)-[6-amino-6-deoxy-α-D-glucopyranosyl-(1→4)]-2-deoxy-D-streptamine monosulfate monohydrate; kanamycin A sulfate hydrate (1:1:1); CAS Reg. N° 58207-20-8.
Description. White or almost white, crystalline powder.
Solubility. Freely soluble in water, practically insoluble in acetone R or ethanol (~750 g/l) TS.
Category. Antibacterial.
Storage. Kanamycin monosulfate should be kept in a tightly closed container, or if sterile, in a hermetically closed container.
Labelling. The label states:
- the content in terms of IU per mg, calculated with reference to the dried substance,
- where applicable, that the substances is free from bacterial endotoxins,
- where applicable, that the substance is sterile.
Requirements
Definition. Kanamycin monosulfate is produced by the growth of certain strains of Streptomyces kanamyceticus. Kanamycin monosulfate contains not less than 750 International Units per mg, with reference to the dried substance.
Manufacture. Kanamycin monosulfate is produced by methods of manufacture designed to eliminate or minimize substances lowering blood pressure.
Identity tests
• Either tests A and D or tests B, C and D may be applied.
A. Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography, using silica gel R5 as the coating substance and a 0.75 M phosphate buffer pH 7.0 as the mobile ate R until pH 7.0 is reached. phase. Prepare the 0.75 M phosphate buffer pH 7.0 by mixing 0.75 M potassium dihydrogen phosphate R with 0.75 M dipotassium hydrogen phosphate R until pH 7.0 is reached.
Apply separately to the plate 2 µl of each of the following solutions in water R. For solution (A) use 5 mg of the test substance per mL. For solution (B) use 5 mg of kanamycin monosulfate RS per mL. For solution (C) use a mixture of 5 mg of kanamycin monosulfate RS and 5 mg of neomycin sulfate RS per mL. After removing the plate from the chromatographic chamber, heat it at 110°C for 5 minutes, spray it with triketohydrindene/methanol reagent TS and heat further at 110°C for 15 minutes.
The principal spot obtained with solution A corresponds in position, appearance, and intensity with that obtained with solution B. The test is not valid unless the chromatogram obtained with solution C shows two clearly separated spots.
B. Dissolve 10 mg in 1 mL of water R, add 1 mL of sodium hydroxide (~80 g/l) TS and mix, then add 2 mL of cobalt(II) nitrate (10 g/l) TS; a greyish colour with precipitate is produced.
C. Dissolve 0.05 g in 3 mL of water R and add 4 mL of anthrone TS; a bluish violet colour is produced.
D. Complies with the test for Sulfate.
Specific optical rotation (1.4). Use a 10 mg/mL solution and calculate with reference to the dried substance;  = +112° to +123°.
= +112° to +123°.
Sulfated ash (2.3). After ignition moisten the residue with 2 mL of nitric acid (~1000 g/l) TS and about 0.2 mL of sulfuric acid (~1760 g/l) TS; not more than 5 mg/g.
Sulfate. 0.15 – 0.17 g/g, calculated as SO4 with reference to the dried substance. Dissolve 0.250 g in 100 mL of water R and adjust the pH of the solution to pH 11 using ammonia (~260 g/l) TS. Add 10.0 mL of barium chloride (0.1 mol/l) VS and 0.5 mg of phthalein purple R. Titrate with disodium edetate (0.1 mol/l) VS adding 50 mL of ethanol (~750 g/l) TS when the colour of the solution begins to change and continue the titration until the violet-blue colour disappears. Each mL of barium chloride (0.1 mol/l) VS is equivalent to 9.606 mg of SO4.
Loss on drying. Dry at 60 °C for 3 hours under reduced pressure (not exceeding 0.6 kPa or about 5 mm mercury); it loses not more than 15 mg/g.
pH value (1.13). pH of a 10 mg/mL solution in carbon-dioxide-free water R, 6.5-8.5.
Bacterial endotoxins. If intended for use in the manufacture of a parenteral dosage form, carry out the test as described under 3.4 Test for bacterial endotoxins; contains not more than 0.67 IU of endotoxin per mg of kanamycin.
Sterility. If intended for use in the manufacture of either a parenteral or other sterile dosage form without a further appropriate sterilization procedure, complies with 3.2 Test for sterility.
Related substances. Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography, using silica gel R5 as the coating substance and a 0.75 M phosphate buffer pH 7.0 as the mobile phase. Prepare the 0.75 M phosphate buffer pH 7.0 by mixing 0.75 M potassium dihydrogen phosphate R with 0.5 M dipotassium hydrogen phosphate R until pH 7.0 is reached.
Apply separately to the plate 5 µl of each of the following solutions in water R. For solution (1) use 10 mg of the test substance per mL. For solution (2) use 0.5 mg of the test substance per mL. For solution (3) use 0.3 mg of the test substance per mL. After removing the plate from the chromatographic chamber, heat it at 110°C for 5 minutes, spray it with triketohydrindene/methanol reagent TS and heat further at 110°C for 15 minutes. For solution (4), use a mixture of 5 mg of kanamycin monosulfate RS and 5 mg of neomycin sulfate RS per mL.
Any spot obtained with solution (1), other than the principal spot is not more intense than that obtained with solution (2) (5.0%) and not more than one such spot is more intense than that obtained with solution (3) (3.0%). The test is not valid unless the chromatogram obtained with solution (4) shows two clearly separated spots.
Assay. Carry out the assay as described under 3.1 Microbiological assay of antibiotics, using either (a) Bacillus subtilis (ATCC 6633) as the test organism, culture medium Cm1 with a final pH of 7.9, sterile phosphate buffer pH 8.0 TS1, an appropriate concentration of kanamycin monosulfate(usually 5-20 IU per mL), and an incubation temperature of 30-37°C, or (b) Staphylococcus aureus (ATCC 6538 P) as the test organism, the same culture medium and phosphate buffer, an appropriate concentration of kanamycin monosulfate (usually 10 IU per mL), and an incubation temperature of 35-39 °C. The precision of the assay is such that the fiducial limits of error of the estimated potency (P = 0.95) are not less than 95% and not more than 105% of the estimated potency. The upper fiducial limit of error of the estimated potency (P = 0.95) is not less than 750 IU per mg, calculated with reference to the dried substance.
Impurities
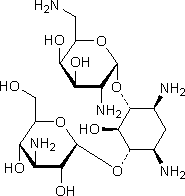
A. 4-O-(3-amino-3-deoxy-α-D-glucopyranosyl)-6-O-(2,6-diamino-2,6-dideoxy-α-D-glucopyranosyl)-2-deoxy-L-streptamine (kanamycin B).