Monographs: Pharmaceutical substances: Lamivudine (Lamivudinum)
Molecular formula. C8H11N3O3S
Relative molecular mass. 229.3
Graphic formula
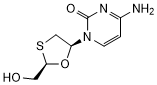
Chemical name. (-)-4-Amino-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2(1H)-one; CAS Reg. No. 134678-17-4.
Description. A white or almost white powder.
Solubility. Soluble in water; sparingly soluble in methanol R; slightly soluble in dehydrated ethanol R; practically insoluble in acetone R.
Category. Antiretroviral (Nucleoside/Nucleotide Reverse Transcriptase Inhibitor).
Storage. Lamivudine should be kept in a well-closed container, protected from light.
Additional information. Lamivudine may exhibit polymorphism.
Requirements
Definition. Lamivudine contains not less than 97.5% and not more than 102.0% ("Assay", Method A) or not less than 97.0% and not more than 103.0% ("Assay", Method B) of C8H11N3O3S, calculated with reference to the dried substance.
Identity tests
-
Either tests A and E, or any two of tests B, C or D together with test E may be applied.
-
Carry out the examination as described under 1.7 Spectrophotometry in the infrared region. The infrared absorption spectrum is concordant with the spectrum obtained from lamivudine RS or with the reference spectrum of lamivudine.
If the spectra thus obtained are not concordant, repeat the test using the residues obtained by separately dissolving the test substance and lamivudine RS in a small amount of methanol R and evaporating to dryness. The infrared absorption spectrum is concordant with the spectrum obtained from lamivudine RS.
-
Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography, using silica gel R6 as the coating substance and a mixture of 67 volumes of dichloromethane R, 20 volumes of acetonitrile R, 10 volumes of methanol R and 3 volumes of ammonia (~260 g/L) TS as the mobile phase. Apply separately to the plate 10 μL of each of the following two solutions in methanol containing (A) 1 mg of the test substance per mL and (B) 1 mg of lamivudine RS per mL. After removing the plate from the chromatographic chamber, allow it to dry in a current of air. Examine the chromatogram in ultraviolet light (254 nm). The principal spot obtained with solution A corresponds in position, appearance and intensity to that obtained with solution B.
Spray the plate with vanillin/sulfuric acid TS1 and heat it for a few minutes at 120 °C. Examine the chromatogram in daylight. The principal spot obtained with solution A corresponds in position, appearance and intensity to that obtained with solution B.
-
The absorption spectrum (1.6) of the final solution prepared for "Assay", Method B, when observed between 210 nm and 300 nm, exhibits one maximum at about 280 nm.
Alternatively, in combination with identity tests D or E.2, where a diode array detector is available, record the UV spectra of the principal peaks in the chromatograms with a diode array detector in the range of 210 nm to 300 nm. The UV spectrum of the principal peak in the chromatogram obtained with solution (1) corresponds to the UV spectrum of the peak due to lamivudine in the chromatogram obtained with solution (2).
-
Carry out the test as described under 1.14.1 Chromatography, High-performance liquid chromatography, using the conditions given under "Assay", Method A. The retention time of the principal peak in the chromatogram obtained with solution (1) corresponds to the retention time of the peak due to lamivudine in the chromatogram obtained with solution (2).
-
Carry out test E.1 or, where a HPLC and the indicated chiral columns are available, test E.2.
E.1 Determine the specific optical rotation (1.4) using a 10 mg/mL solution in methanol R and calculate with reference to the dried substance;
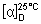 = -135 to -144.
= -135 to -144.E.2 Carry out the test as described under 1.14.1 Chromatography, High-performance liquid chromatography, using the conditions and solutions given under "Impurity D (lamivudine enantiomer)". The retention time of the principal peak obtained with solution (1) corresponds to the retention time of the peak due to lamivudine in the chromatogram obtained with solution (2).
Heavy metals. Use 1.000 g of the test substance for the preparation of the test solution as described under 2.2.3 Limit test for heavy metals, procedure 4. Determine the heavy metals content according to method A; not more than 20 μg/g.
Sulfated ash (2.3). Not more than 1.0 mg/g.
Loss on drying. Dry 1.000 g of the test substance for 3 hours at 105 °C; it loses not more than 5 mg/g.
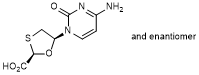
Impurity D (lamivudine enantiomer). Carry out the test as described under 1.14.1 Chromatography, High-performance liquid chromatography, using a stainless steel column (25 cm x 4.6 mm) packed with particles of silica gel, the surface of which has been modified with chemically-bonded 2-hydroxypropyl-β-cyclodextrin, (5 µm). As mobile phase, use a mixture of 5 volumes of methanol R and 95 volumes of a 7.7 g/L solution of ammonium acetate R.
Operate at a flow rate of 1.0 mL per minute. As a detector, use an ultraviolet spectrophotometer set at a wavelength of 270 nm. Maintain the column temperature between 15 °C and 30 °C. The temperature may be adjusted to optimize the resolution between lamivudine and impurity D; a lower temperature may improve resolution.
Prepare the following solutions in water. For solution (1), dissolve 25.0 mg of the test substance and dilute to 100.0 mL. For solution (2), dissolve the content of a vial lamivudine for system suitability 2 RS (containing lamivudine and impurity D) and dilute to 1.0 mL.
Inject 10 µL each of solutions (1) and (2). Record the chromatograms for about twice the retention time of lamivudine.
The impurities, if present, are eluted at the following relative retentions with reference to lamivudine (retention time about 8 minutes): impurity D (lamivudine enantiomer) about 1.2; impurity B and enantiomer about 1.3 and 1.5.
The test is not valid unless the peak-to-valley ratio (Hp/Hv) is at least 15, where Hp is the height above the baseline of the peak due to impurity D and Hv is the height above the baseline of the lowest point of the curve separating this peak from the peak due to lamivudine.
Calculate the sum of the percentage contents of impurity B and enantiomer and impurity D. Subtract the percentage content of impurity B as obtained in the test for related substances. The concentration of impurity D is not greater than 0.3%.
Related substances. Carry out the test as described under 1.14.1 Chromatography, High-performance liquid chromatography, using a stainless steel column (25cm x 4.6mm) packed with base deactivated particles of silica gel, the surface of which has been modified with chemically bonded octadecylsilyl groups (5 μm). As the mobile phase, use a mixture of 5 volumes of methanol R and 95 volumes of an acetate buffer pH 3.8. Prepare the buffer by dissolving 1.9 g of ammonium acetate R in 900 mL of water R, adjust to pH 3.8 with glacial acetic acid R and dilute to 1000 mL.
Prepare the following solutions in mobile phase. For solution (1), dissolve 50.0 mg of the test substance and dilute to 100.0 mL. For solution (2), dilute 1.0 mL of solution (1) to 100 mL. For solution (3), dilute 1.0 mL of solution (2) to 10.0 mL. For solution (4), dissolve 5 mg of salicylic acid R in 100.0 mL. Dilute 1.0 mL of this solution to 100.0 mL. For solution (5), dissolve 5 mg of cytosine R and 5 mg of uracil R and dilute to 100.0 mL. Dilute 2.0 mL of this solution to 10.0 mL. For solution (6), dissolve 5 mg of lamivudine for system suitability 1 RS (containing lamivudine and lamivudine impurities A and B) in 2 mL, add 1 mL of solution (5) and dilute to 10 mL.
Operate with a flow rate of 1.0 mL per minute. As a detector, use an ultraviolet spectrophotometer set at a wavelength of about 277 nm. For identity test C.2, use a diode array detector in the range of 210 to 300 nm. Maintain the temperature of the column at 35 °C.
Inject 10 μl each of solutions (1), (2), (3), (4) and (6).
Record the chromatograms for about three times the retention time of lamivudine. Use the chromatograms obtained with solutions (4) and (6) to identify the peaks due to impurities A, B, C, E and F. The impurity peaks are eluted at the following relative retention with reference to lamivudine (retention time about 9 minutes): impurity E about 0.28; impurity F about 0.32; impurity A about 0.36; impurity B about 0.91; impurity J about 1.45; impurity C about 2.32.
The test is not valid unless, in the chromatogram obtained with solution (6), the resolution factor between the peaks due to impurity B and lamivudine is at least 1.5. Also, the test is not valid unless, in the chromatogram obtained with solution (3), the peak due to lamivudine is detected with a signal-to-noise ratio of at least 25.
In the chromatogram obtained with solution (1):
-
the area of any peak corresponding to impurity A is not greater than three times the area of the peak due to lamivudine in the chromatogram obtained with solution (3) (0.3%);
-
the area of any peak corresponding to impurity B is not greater than twice the area of the peak due to lamivudine in the chromatogram obtained with solution (3) (0.2%);
-
the area of any peak corresponding to impurity C is not greater than the peak due to salicylic acid in the chromatogram obtained with solution (4) (0.1%);
-
the area of any peak corresponding to impurity E, when multiplied by a correction factor of 0.6, is not greater than the area of the peak due to lamivudine in the chromatogram obtained with solution (3) (0.1%);
-
the area of any peak corresponding to either impurities F or J, when multiplied by a correction factor of 2.2, is not greater than the area of the peak due to lamivudine in the chromatogram obtained with solution (3) (0.1%);
-
the area of any other impurity peak is not greater than the area of the peak due to lamivudine in the chromatogram obtained with solution (3) (0.1%).
-
The sum of the areas of all impurity peaks, including the corrected areas of any peaks corresponding to impurities E, F or J, is not greater than 0.6 times the area of the peak due to lamivudine in the chromatogram obtained with solution (2) (0.6%). Disregard any peak with an area less than 0.5 times the area of the principal peak obtained with solution (3) (0.05%).
Assay
-
Either method A or method B may be applied.
-
Carry out the test as described under 1.14.1 Chromatography, High-performance liquid chromatography, using the conditions given under "Related substances" with the following modifications.
Use solution (1) as described under "Related substances". For solution (2), dissolve 50.0 mg of lamivudine RS in mobile phase and dilute to 100.0 mL with the same solvent.
Inject 10 μL each of solutions (1) and (2).
Measure the areas of the peaks corresponding to lamivudine obtained in the chromatograms of solutions (1) and (2) and calculate the percentage content of C8H11N3O3S, using the declared content of C8H11N3O3S in lamivudine RS.
-
Transfer 50.0 mg of the test substance into a 500 mL volumetric flask and dissolve in about 400 mL of water R. Sonicate, if necessary. Cool to room temperature, dilute to volume with water R and mix. Dilute 5.0 mL of this solution to 50.0 mL with sulfuric acid (0.1 mol/L) VS and mix.
Measure the absorbance (1.6) of a 1.0 cm layer of the final solution at the maximum of about 280 nm using a solution prepared by mixing 5 mL of water R with 50 mL of sulfuric acid (0.1 mol/L) VS as a blank. Calculate the content of C8H11N3O3S using the absorptivity value of 60.7 (
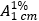 = 607).
= 607).
Impurities
A. (2RS,5SR)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)-1,3-oxathiolane-2-carboxylic acid (lamivudine carboxylic acid) (synthesis related impurity).
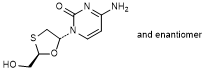
B. 4-amino-1-[(2RS,5RS)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2(1H)-one, (lamivudine diastereomer, (+/-)-trans-lamivudine) (synthesis related impurity).
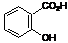
C. 2-hydroxybenzoic acid (salicylic acid) (synthesis related impurity).
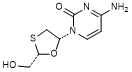
D. (+)-4-amino-1-[(2S,5R)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2(1H)-one (ent-lamivudine) (synthesis related impurity).
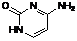
E. 4-aminopyrimidin-2(1H)-one (cytosine) (synthesis related impurity, degradation product).
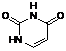
F. pyrimidine-2,4(1H,3H)-dione (uracil) (synthesis related impurity, degradation product).
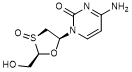
G. 4-amino-1-[(2R,3S,5S)-2-(hydroxymethyl)-3-oxo-1,3λ4-oxathiolan-5-yl]pyrimidin-2(1H)-one (lamivudine S-sulfoxide) (synthesis related impurity, degradation product).
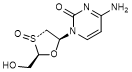
H. 4-amino-1-[(2R,3R,5S)-2-(hydroxymethyl)-3-oxo-1,3λ4-oxathiolan-5-yl]pyrimidin-2(1H)-one (lamivudine R-sulfoxide) (synthesis related impurity, degradation product).
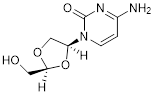
I. (+)-4-amino-1-[(2S,4S)-2-(hydroxymethyl)-1,3-dioxolan-4-yl]pyrimidin-2(1H)-one.
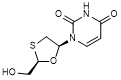
J. 1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidine-2,4(1H,3H)-dione; 1-[(2R,5S)-2-(hydroxmethyl)-1,3-oxathiolan-5yl]uracil (lamivudine uracil derivative) (degradation product).