Monographs: Pharmaceutical substances: Levamisole hydrochloride (Levamisoli hydrochloridum)
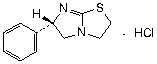
C11H12N2S.HCl
Relative molecular mass. 240.8
Chemical name. (6S)-6-phenyl-2,3,5,6-tetrahydroimidazo[2,1-b][1,3]thiazole monohydrochloride; (6S)-2,3,5,6-tetrahydro-6-phenylimidazo[2,1-b]thiazole hydrochloride (1:1); CAS Reg. No. 16595-80-5.
Description. A white or almost white, crystalline powder.
Solubility. Freely soluble in water; soluble in ethanol (~750 g/L) TS; slightly soluble in dichloromethane R.
Category. Anthelminthic.
Storage. Levamisole hydrochloride should be kept in a well-closed container, protected from light.
Requirements
Levamisole hydrochloride contains not less than 98.5% and not more than 101.0% of C11H12N2S,HCl, calculated with reference to the dried substance.
Identity tests
- Either tests A, D and E or tests B, C, D and E may be applied.
A. Carry out the examination as described under 1.7 Spectrophotometry in the infrared region. The infrared absorption spectrum is concordant with the spectrum obtained from levamisole hydrochloride RS or with the reference spectrum of levamisole hydrochloride.
B. Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography using silica gel R6 as the coating substance and a mixture of 60 volumes of toluene R, 40 volumes of acetone R and 1 volume of ammonia (~260 g/L) TS as the mobile phase. Apply separately to the plate 10 μL of each of the following two solutions in methanol R. For solution (A) use 2 mg of the test substance per mL. For solution (B) use 2 mg of levamisole hydrochloride RS per mL. Examine the chromatogram in ultraviolet light (254 nm). The principal spot obtained with solution (A) corresponds in position, appearance and intensity with that obtained with solution (B).
C. Dissolve about 60 mg of the test substance in 20 mL of water, add 2 mL of sodium hydroxide (~80 g/L) TS, boil for 10 minutes and cool. Add a few drops of sodium nitroprusside (45 g/L) TS; a red colour is produced which fades on standing.
D. A 50 mg/mL solution yields reaction B described under 2.1 General identification tests as characteristic of chlorides.
E. Determine the specific optical rotation (1.4). Use a 0.050 g/mL solution in carbon dioxide-free water R and calculate with reference to the dried substance;
= -121° to -128°.
Clarity and colour of solution. A solution of 0.50 g in 10 mL of carbon dioxide-free water R is clear and not more intensely coloured than standard colour Yw1 when compared as described under 1.11.1 Colour of liquids.
Sulfated ash. Not more than 1.0 mg/g.
Loss on drying. Dry to constant mass at 105 °C; it loses not more than 5.0 mg/g.
pH value. pH of a 50 mg/mL solution, 3.0–4.5.
Related substances. Prepare fresh solutions and perform the tests without delay.
Carry out the test as described under 1.14.1 Chromatography, High-performance liquid chromatography using a stainless steel column (10 cm x 4.6 mm) packed with particles of base-deactivated silica gel, the surface of which has been modified by chemically-bonded octadecylsilyl groups (3 µm).
Use the following conditions for gradient elution:
mobile phase A: dissolve 0.5 g of ammonium dihydrogen phosphate R in 90 mL of water R, adjust to pH 6.5 with sodium hydroxide (~40 g/L) TS and dilute to 100 mL with water R;
mobile phase B: acetonitrile R.
| Time (min) |
Mobile phase A (% v/v) |
Mobile phase B (% v/v) |
Comments |
|
0–8 |
90–30 |
10–70 |
Linear gradient |
|
8–10 |
30 |
70 |
Isocratic |
|
10–11 |
30–90 |
70–10 |
Return to initial composition |
|
11–15 |
90 |
10 |
Re-equilibration |
Operate with a flow rate of 1.5 mL per minute. As a detector use an ultraviolet spectrophotometer set at a wavelength of 215 nm.
As a dissolution solvent prepare a mixture of 90 volumes of methanol R and 10 volumes of ammonia (~260 g/L) TS. Prepare the following solutions: for solution (1) transfer 100 mg of the test substance to a 10 mL volumetric flask, dissolve in about 6 mL of the dissolution solvent and dilute to volume with the dissolution solvent. For solution (2) dilute 1.0 mL of solution (1) to 100.0 mL with methanol R. For solution (3) dissolve in a test tube 20 mg of the test substance in 5 mL of a 0.1 mol/L solution of sodium hydroxide R. Close and heat the test tube in a boiling water-bath for 5 hours. Allow to cool and dilute 1 mL of the resulting solution to 25 mL with methanol R. For solution (4) transfer 5.0 mL of solution (2) to a 50 mL volumetric flask and dilute to volume with methanol.
Inject 10 µL of solution (3). The test is not valid unless the chromatogram shows a peak due to impurity C eluting at the relative retention of about 1.5 with reference to levamisole (retention time about 3.5 minutes). Inject separately 10 µL each of solutions (1) and (4).
The chromatogram obtained with solution (1) may show peaks due to the following impurities eluting at the following relative retention with reference to levamisole (retention time about 3.5 minutes): impurity A about 0.9; impurity B about 1.4; impurity C about 1.5; impurity D about 1.6; impurity E about 2.0.
In the chromatogram obtained with solution (1):
- the area of any peak corresponding to impurity A, when multiplied by a correction factor of 2.0, is not greater than 2 times the area of the peak due to levamisole in the chromatogram obtained with solution (4) (0.2%);
- the area of any peak corresponding to impurity B, when multiplied by a correction factor of 1.7, is not greater than 2 times the area of the peak due to levamisole in the chromatogram obtained with solution (4) (0.2%);
- the area of any peak corresponding to impurity C, when multiplied by a correction factor of 2.9, is not greater than 2 times the area of the peak due to levamisole in the chromatogram obtained with solution (4) (0.2%);
- the area of any peak corresponding to impurity D, when multiplied by a correction factor of 1.3, is not greater than 2 times the area of the peak due to levamisole in the chromatogram obtained with solution (4) (0.2%);
- the area of any peak corresponding to impurity E, when multiplied by a correction factor of 2.7, is not greater than 2 times the area of the peak due to levamisole in the chromatogram obtained with solution (4) (0.2%);
- the area of any other peak, other than the principal peak, is not greater than the area of the peak due to levamisole in the chromatogram obtained with solution (4) (0.1%);
- the sum of the corrected areas of any peak corresponding to impurity A, B, C, D and E and the areas of all other peaks, other than the principal peak, is not greater than 3 times the area of the peak due to levamisole in the chromatogram obtained with solution (4) (0.3%). Disregard any peak with an area less than 0.5 times the area of the peak due to levamisole the chromatogram obtained with solution (4) (0.05%).
Heavy metals. Use 1.0 g for the preparation of the test solution as described under 2.2.3 Limit test for heavy metals, use procedure 1 or procedure 3 for the preparation of the test solution; determine the heavy metals content according to method A; not more than 20 μg/g.
Assay
Dissolve about 0.2 g, accurately weighed, in 30 mL of ethanol (~750 g/L) TS and add 5 mL of hydrochloric acid (0.01 mol/L) VS. Titrate with sodium hydroxide (0.1 mol/L) VS, determining the two inflection points potentiometrically. Record the volume in mL of sodium hydroxide (0.1 mol/L) VS consumed between the two inflection points.
Each mL of sodium hydroxide (0.1mol/L) VS is equivalent to 24.08 mg of C11H12N2S,HCl.
Impurities

A. rac-3-[(2R)-2-amino-2-phenylethyl]-1,3-thiazolidin-2-one,
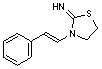
B. 3-[(E)-2-phenylethenyl]thiazolidin-2-imine,
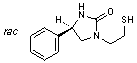
C. rac-(4R)-4-phenyl-1-(2-sulfanylethyl)imidazolidin-2-one,
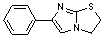
D. 6-phenyl-2,3-dihydroimidazo[2,1-b][1,3]thiazole,
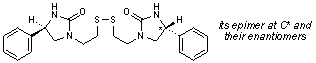
E. 1,1'-[disulfanediylbis(ethane-2,1-diyl)]bis[(4RS)-4-phenylimidazolidin-2-one].