Monographs: Pharmaceutical substances: Levofloxacin hemihydrate (Levofloxacinum hemihydricum)
Molecular formula. C18H20FN3O4. ½ H2O
Relative molecular mass. 370.4
Graphic formula.
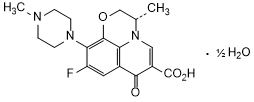
Chemical name. (3S)-9-Fluoro-3-methyl-10-(4-methylpiperazin-1-yl)-7-oxo-2,3-dihydro-7 H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid hemihydrate; CAS Reg. No. 138199-71-0.
Description. Light yellowish-white or slightly yellow powder.
Solubility. Sparingly soluble in water R, freely soluble in acetic acid (~300 g/L) TS, sparingly soluble in methanol R, and slightly soluble in dehydrated ethanol R.
Category. Antibacterial, antituberculosis.
Storage. Levofloxacin hemihydrate should be kept in a tightly closed container, protected from light.
Labelling. The designation on the container should state the substance is in the form of the hemihydrate.
Additional information. Levofloxacin may exhibit polymorphism.
Requirements
Definition. Levofloxacin hemihydrate contains not less than 98.0% and not more than 101.0% of levofloxacin (C18H20FN3O4) calculated with reference to the anhydrous substance.
Identity test
Either tests A and D or tests B and C may be applied.
-
Carry out the examination as described under 1.7 Spectrophotometry in the infrared region. The infrared absorption spectrum is concordant with the spectrum obtained from levofloxacin RS or with the reference spectrum of levofloxacin hemihydrate.
If the spectra thus obtained are not concordant, repeat the test using the residues obtained by separately dissolving the test substance and levofloxacin RS in a small amount of acetonitrile R and evaporating to dryness. The infrared absorption spectrum is concordant with the spectrum obtained from levofloxacin RS.
-
Carry out the test as described under 1.14.1. Thin layer chromatography, using silica gel R5 as the coating substance and a mixture of 4 volumes of 1-butanol R, 4 volumes of methanol R and 2 volumes of ammonia (~100 g/L) TS as the mobile phase. Apply separately to the plate 5 mL of each of the following two solutions in a mixture of 1 volume of methanol R and 4 volumes of dichloromethane R. For solution (A), use a solution containing 5 mg of the test substance per mL. For solution (B), use a solution containing 5 mg of levofloxacin RS per mL. After removing the plate from the chromatographic chamber, allow it to dry exhaustively in air or in a current of cool air. Examine the chromatogram in ultraviolet light (366 nm).
The principal spot obtained with solution (A) corresponds in position, appearance, and intensity with the spot due to levofloxacin in the chromatogram obtained with solution (B).
-
Carry out the test as described under 1.14.1 Chromatography, High-performance liquid chromatography using the conditions given under “Related substances” with the following modifications. For solution (1) use solution (1) as described under “Related substances”. For solution (2), dissolve 50.0 mg of levofloxacin hydrochloride RS in 50.0 mL mobile phase. Inject 5 µL of solution (1) and (2). The retention time of the principal peak in the chromatogram obtained with solution (1) corresponds to the retention time of the peak due to levofloxacin in the chromatogram obtained with solution (2).
-
Determine the specific optical rotation (1.4) using a solution containing 5.0 mg of the test substance per mL methanol R and calculate with reference to the anhydrous substance;
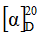 = -92 to -106.
= -92 to -106.
Heavy metals . Use 2.0 g for the preparation of the test solution as described under 2.2.3 Limit test for heavy metals, Procedure 3. Determine the heavy metals content according to Method A; not more than 10 μg/g.
Sulfated ash (2.3). Not more than 1.0 mg/g.
Water. Determine as described under 2.8 Determination of water by Karl Fischer Method, Method A. Use 0.500 g of the test substance and a mixture of equal volumes of formamide R and methanol R as solvent. The water content is not less than 20 mg/g and not more than 30 mg/g.
Related substances. Prepare fresh solutions protected from light and perform the test without delay.
Carry out the test as described under 1.14.1 Chromatography, High-performance liquid chromatography using a stainless steel column (25 cm x 4.6 mm), packed with end-capped and base-deactivated particles of silica gel, the surface of which has been modified with chemically-bonded octadecylsilyl groups (5 μm).
Prepare the following buffer solution. Dissolve 1.25 g of copper (II) sulfate pentahydrate R, 1.3 g of isoleucine R and 8.5 g ammonium acetate R in water R and dilute to 1000 mL with the same solvent.
As the mobile phase, use a mixture of methanol R and the buffer solution (30:70 v/v). Operate with a flow rate of 0.8 mL per minute. As a detector, use an ultraviolet spectrophotometer set at a wavelength of 360 nm. Maintain the column at a temperature of 45 °C.
Prepare the following solutions in mobile phase. For solution (1), dissolve 50.0 mg of the test substance in 50.0 mL. For solution (2), dilute 1.0 mL of solution (1) to 100.0 mL. For solution (3), dilute 1.0 mL of solution (2) to 10.0 mL. For solution (4), use a solution containing 1.0 mg of levofloxacin for system suitability RS (containing levofloxacin and the impurities A, B and G) per mL.
Inject alternately 25 µL of solution (1), (2), (3) and (4). Record the chromatogram for three times the retention time of levofloxacin.
Use the chromatogram obtained with solution (4) and the chromatogram supplied with levofloxacin for system suitability RS to identify the peaks due to the impurities A, B and G. The impurities are eluted at the following relative retention with reference to levofloxacin (retention time about 20 minutes); impurity B about 0.50; impurity G about 0.56; impurity A about 1.22.
The test is not valid unless, in the chromatogram obtained with solution (4), the resolution between the peaks due to impurity B and the peak due to impurity G is at least 1.5.
In the chromatogram obtained with solution (1):
- the area of any peak corresponding to impurity B, when multiplied by a correction factor of 1.3, is not greater than three times the area of the peak due to levofloxacin in the chromatogram obtained with solution (3) (0.3 %);
- the area of any peak corresponding to impurity G, when multiplied by a correction factor of 1.1, is not greater than three times the area of the peak due to levofloxacin in the chromatogram obtained with solution (3) (0.3 %);
- the area of any peak corresponding to impurity A is not greater than the area of the peak due to levofloxacin in the chromatogram obtained with solution (2) (1.0 %);
- the area of any other impurity peak is not greater than the area of the peak due to levofloxacin in the chromatogram obtained with solution (3) (0.10 %).
- The sum of the corrected areas of any peak corresponding to impurity B or G and the areas of all other impurity peaks, other than any peak due to impurity A, is not greater than 0.5 times the area of the peak due to levofloxacin in the chromatogram obtained with solution (2) (0.5 %). Disregard any peak with an area less than 0.5 times the area of the peak due to levofloxacin in the chromatogram in the chromatogram obtained with solution (3) (0.05%).
Impurity F. Carry out the test as described under 1.14.1 Chromatography, High-performance liquid chromatography using the conditions given under “Related substances” with the following modifications. As the mobile phase, use a mixture of methanol R and the buffer solution (50:50 v/v). As a detector, use an ultraviolet spectrophotometer set at a wavelength of 320 nm.
Prepare the following solutions in mobile phase. For solution (1), dissolve 50.0 mg of the test substance in 50.0 mL. For solution (2), dissolve 5.0 mg of levofloxacin impurity F RS and dilute to 100.0 mL. For solution (3), dilute 1.0 mL of solution (2) to 25.0 mL. For solution (4), dilute 4.0 mL of solution (2) to 10.0 mL. Dilute 1.0 mL of this solution to 10.0 mL with solution (1).
Inject alternately 25 µL of solution (1), (3) and (4). Record the chromatogram for three times the retention time of levofloxacin.
Use the chromatogram obtained with solution (3) to identify the peaks due to impurity F. Impurity F, is eluted at the relative retention of 1.8 with reference to levofloxacin (retention time: about 6 minutes).
The test is not valid unless, in the chromatogram obtained with solution (4), the resolution between the peak due to impurity F and the peak due to levofloxacin is at least 5.
Measure the areas of the peaks corresponding to impurity F obtained in the chromatograms of solution (1) and (3) and calculate the percentage content of impurity F, using the concentration of impurity F in solution (3). The concentration of impurity F is not more than 0.2%.
Assay. Prepare fresh solutions protected from light and perform the test without delay.
Dissolve 0.300 g in 100 mL of glacial acetic acid R and titrate with perchloric acid (0.1 mol/L) VS as described under 2.6. Non-aqueous titrations, Method A determining the end point potentiometrically. Each ml of perchloric acid (0.1 mol/L) VS is equivalent to 36.14 mg of C18H20FN3O4.
Impurities
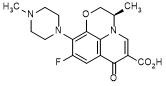
A. (3R )-9-fluoro-3-methyl-10-(4-methylpiperazin-1-yl)-7-oxo-2,3-dihydro-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid (dextrofloxacin, synthesis-related impurity),
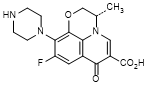
B. (3S)-9-fluoro-3-methyl-7-oxo-10-(piperazin-1-yl)-2,3-dihydro-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid (N-desmethyl levofloxacin),
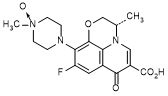
C. 4-[(3S)-6-carboxy-9-fluoro-3-methyl-7-oxo-2,3-dihydro-7H-pyrido[1,2,3-de]-1,4-benzoxazin-10-yl]-1-methylpiperazine 1-oxide (levofloxacin N-oxide; degradation product),
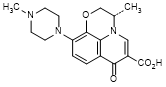
D. (3S)-3-methyl-10-(4-methylpiperazin-1-yl)-7-oxo-2,3-dihydro-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid (9-desfluoro levofloxacin, synthesis related impurity),
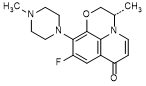
E. (3S)-9-fluoro-3-methyl-10-(4-methylpiperazin-1-yl)-2,3-dihydro-7H-pyrido[1,2,3-de]-1,4-benzoxazin-7-one (decarboxy levofloxacin, synthesis related impurity),
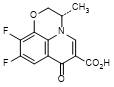
F. (3S)-9,10-difluoro-3-methyl-7-oxo-2,3-dihydro-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid,
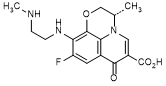
G. (3S)-9-fluoro-3-methyl-10-{[2-(methylamino)ethyl]amino}-7-oxo-2,3-dihydro-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid (diamine derivative)
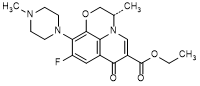
H. ethyl (3S)-9-fluoro-3-methyl-10-(4-methylpiperazin-1-yl)-7-oxo-2,3-dihydro-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylate
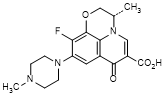
I. (3S)-10-fluoro-3-methyl-9-(4-methylpiperazin-1-yl)-7-oxo-2,3-dihydro-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid (levofloxacin 9-piperazino isomer, synthesis related impurity)