Monographs: Pharmaceutical substances: Levodopa (Levodopum)
Molecular formula. C9H11NO4
Relative molecular mass. 197.2
Graphic formula.
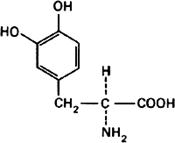
Chemical name. (-)-3-(3,4-Dihydroxyphenyl)-L-alanine; 3-hydroxy-L-tyrosine; CAS Reg. No. 59-92-7.
Description. A white or almost white, crystalline powder; odourless.
Solubility. Soluble in 300 parts of water; practically insoluble in ethanol (~750 g/l) TS and ether R.
Category. Antiparkinsonism drug.
Storage. Levodopa should be kept in a tightly closed container.
Requirements
Definition. Levodopa contains not less than 98.5% and not more than 101.0% of C9H11NO4, calculated with reference to the dried substance.
Identity tests
• Either test A alone or tests B and C may be applied.
A. Carry out the examination as described under 1.7 Spectrophotometry in the infrared region. The infrared absorption spectrum is concordant with the spectrum obtained from levodopa RS or with the reference spectrum of levodopa.
B. See the test described below under "Related substances". The principal spot obtained with solution B corresponds in position, appearance, and intensity with that obtained with solution C.
C. To 5 mg add 1 mL of water, 1 mL of pyridine R and 5 mg of 4-nitrobenzoyl chloride R, mix and allow to stand for 3 minutes; a violet colour is produced which changes to pale yellow on boiling. While shaking, add 0.1 mL of sodium carbonate (200 g/l) TS; the violet colour reappears.
Specific optical rotation. Transfer about 0.5 g, accurately weighed, to a 25-mL volumetric flask, dissolve it in 10 mL of hydrochloric acid (1 mol/l) VS, add 5 g of methenamine R, swirl the contents to dissolve the methenamine, dilute with sufficient hydrochloric acid (1 mol/l) VS to produce 25 mL, mix, and allow to stand in the dark at 25°C for 3 hours;  = -160° to -167°.
= -160° to -167°.
Heavy metals. Use 1.0 g for the preparation of the test solution as described under 2.2.3 Limit test for heavy metals, Procedure 3; determine the heavy metals content according to Method A; not more than 10 μg/g.
Clarity and colour of solution. Dissolve 0.50 g in a mixture of 2 mL of hydrochloric acid (~70 g/L) TS and 8 mL of water; the solution is clear and not more intensely coloured than standard colour solution Yw2 when compared as described under 1.11.1 Colour of liquids.
Sulfated ash. Not more than 1.0 mg/g.
Loss on drying. Dry to constant weight at 105°C; it loses not more than 10 mg/g.
Related substances. Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography, using cellulose R2 as the coating substance and a mixture of 50 volumes of 1-butanol R, 25 volumes of glacial acetic acid R, and 25 volumes of water as the mobile phase. Prepare a fresh solution of 0.10 g of the test substance dissolved in 5 mL of anhydrous formic acid R and dilute to 10 mL with methanol R; this constitutes solution A. Dilute 0.5 mL of solution A to 100 mL with methanol R; this constitutes solution B. Separately prepare a fresh solution of 0.10 g of levodopa RS dissolved in 5 mL of anhydrous formic acid R and dilute to 10 mL with methanol R. Dilute 0.5 mL of this solution to 100 mL with methanol R; this constitutes solution C. Dissolve 30 mg of tyrosine R in 1 mL of anhydrous formic acid R and dilute to 100 mL with methanol R. Mix 1 mL of this solution with 1 mL of solution A; this constitutes solution D. Apply separately to the plate 10 μl of each of solutions A, B, and C, and 20 μl of solution D. Dry the plate in a stream of air before placing it in the chromatographic chamber. Develop the plate, remove it from the chamber, allow it to dry in air, spray with a freshly prepared solution containing 2 volumes of ferric chloride (25 g/l) TS and 1 volume of potassium ferricyanide (50 g/l) TS, and examine the chromatogram immediately in ultraviolet light (365 nm). Any spot obtained with solution A, other than the principal spot, is not more intense than that obtained with solution B. The test is valid only if the chromatogram obtained with solution D shows two distinctly separated spots.
Assay. Dissolve by heating about 0.18 g, accurately weighed, in 5 mL of anhydrous formic acid R, add 25 mL of glacial acetic acid R1 and 25 mL of dioxan R, and titrate with perchloric acid (0.1 mol/l) VS as described under 2.6 Non-aqueous titration. Method A. Each mL of perchloric acid (0.1 mol/l) VS is equivalent to 19.72 mg of C9H11NO4.