Monographs: Pharmaceutical substances: Lidocaine (Lidocainum)
Molecular formula. C14H22N2O
Relative molecular mass. 234.3
Graphic formula.
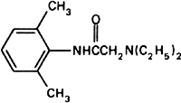
Chemical name. 2-(Diethylamino)-2',6'-acetoxylidide; 2-(diethylamino)-N-(2,6-dimethylphenyl)acetamide; CAS Reg. No. 137-58-6.
Description. A white or slightly yellow, crystalline powder; odour, characteristic.
Solubility. Practically insoluble in water; very soluble in ethanol (~750 g/l) TS; freely soluble in benzene R and ether R.
Category. Local anaesthetic.
Storage. Lidocaine should be kept in a tightly closed container, protected from light.
Additional information. Lidocaine causes local numbness after being placed on the tongue.
Requirements
Definition. Lidocaine contains not less than 99.0% and not more than 101.0% of C14H22N2O, calculated with reference to the dried substance.
Identity tests
• Either test A alone or tests B and C may be applied.
A. Carry out the examination as described under 1.7 Spectrophotometry in the infrared region. The infrared absorption spectrum is concordant with the spectrum obtained from lidocaine RS or with the reference spectrum of lidocaine.
B. Dissolve 0.1 g in 1 mL of ethanol (~750 g/l) TS, add 0.5 mL of cobaltous chloride TS, and shake for 2 minutes; a bluish green precipitate is produced.
C. Dissolve 0.1 g in 15 mL of ethanol (~750 g/l) TS and add 10 mL of trinitrophenol (7 g/l) TS. Filter, wash the precipitate with water, and dry at 105°C. Melting temperature, about 230°C (picrate).
Melting range. 66-69°C.
Heavy metals. For the preparation of the test solution use 1.0 g dissolved in a mixture of 4 mL of hydrochloric acid (~70 g/l) TS and 21 mL of water, and proceed as described under 2.2.3 Limit test for heavy metals, Procedure 1; determine the heavy metals content according to Method A; not more than 20 μg/g.
Chlorides. Dissolve 0.50 g in a mixture of 2 mL of nitric acid (~130 g/l) TS and 20 mL of water, filter if necessary, and proceed as described under 2.2.1 Limit test for chlorides; the chloride content is not more than 0.5 mg/g.
Sulfates. Dissolve 0.50 g in 5 mL of hydrochloric acid (~70 g/l) TS, and proceed as described under 2.2.2 Limit test for sulfates; the sulfate content is not more than 1 mg/g.
Sulfated ash. Not more than 1.0 mg/g.
Loss on drying. Dry to constant weight over phosphorus pentoxide R at ambient temperature; it loses not more than 5.0 mg/g.
Primary aromatic amines. Dissolve 0.10 g in 4 mL of hydrochloric acid (~70 g/L) TS using a 100-mL volumetric flask. Cool the solution in an ice-bath. In a test-tube, dissolve 50 mg of sodium nitrite R in 10 mL of water and cool the solution. To the flask in the ice-bath, add half of the volume of the cooled sodium nitrite solution. Allow to stand for 10 minutes, lift the flask from the ice-bath, and add 1 g of urea R. Shake the flask frequently and when the evolution of gas has ceased (about 15 minutes), add 2.5 mL of sodium hydroxide (~80 g/L) TS in which 10 mg of thymol R have previously been dissolved. Add 5 mL of sodium hydroxide (~80 g/L) TS, allow to stand for 10 minutes and dilute to volume. Prepare a blank as described above, but without the substance being examined. The test solution is not more intensely coloured than the blank solution when compared as described under 1.11.1 Colour of liquids.
Assay. Dissolve about 0.45 g, accurately weighed, in 30 mL of glacial acetic acid R1, and titrate with perchloric acid (0.1 mol/l) VS, as described under 2.6 Non-aqueous titration. Method A. Each mL of perchloric acid (0.1 mol/l) VS is equivalent to 23.43 mg of C14H22N2O.