Monographs: Pharmaceutical substances: Lidocaine hydrochloride (Lidocaini hydrochloridum)
Molecular formula. C14H22N2O,HCl,H2O
Relative molecular mass. 288.8
Graphic formula.
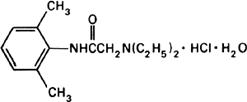
Chemical name. 2-(Diethylamino)-2',6' acetoxylidide monohydrochloride monohydrate; 2-(diethylamino)-N-(2,6-dimethylphenyl)acetamide monohydrochloride monohydrate; CAS Reg. No. 6108-05-0.
Description. A white, crystalline powder; odourless.
Solubility. Soluble in 0.7 part of water and in 1.5 parts of ethanol (~750 g/l) TS; practically insoluble in ether R.
Category. Local anaesthetic.
Storage. Lidocaine hydrochloride should be kept in a tightly closed container, protected from light.
Additional information. Lidocaine hydrochloride causes local numbness after being placed on the tongue. Even in the absence of light, Lidocaine hydrochloride is gradually degraded on exposure to a humid atmosphere, the decomposition being faster at higher temperatures.
Requirements
Definition. Lidocaine hydrochloride contains not less than 99.0% and not more than 101.0% of C14H22N2O,HCl, calculated with reference to the anhydrous substance.
Identity tests
• Either tests A and C or tests B, C, and D may be applied.
A. Carry out the examination as described under 1.7 Spectrophotometry in the infrared region. The infrared absorption spectrum is concordant with the reference spectrum of lidocaine hydrochloride.
B. Dissolve 0.15 g in 10 mL of water, make the solution alkaline with sodium hydroxide (~80 g/l) TS, filter, and wash the precipitate with water. Dissolve the precipitate in 1 mL of ethanol (~750 g/l) TS, add 0.5 mL of cobaltous chloride TS, and shake for 2 minutes; a bluish green precipitate is produced.
C. A 0.05 g/mL solution yields reaction B, described under 2.1 General identification tests as characteristic of chlorides.
D. Dissolve 0.1 g in 10 mL of water and add 10 mL of trinitrophenol (7 g/l) TS. Filter, wash the precipitate with water and dry at 105°C. Melting temperature, about 230°C (picrate).
Melting range. 74-79°C.
Heavy metals. Use 1.0 g for the preparation of the test solution as described under 2.2.3 Limit test for heavy metals, Procedure 1; determine the heavy metals content according to Method A; not more than 10 μg/g.
Clarity and colour of solution. A solution of 1.0 g in 10 mL of water is clear and colourless.
Sulfated ash. Not more than 1.0 mg/g.
Water. Determine as described under 2.8 Determination of water by the Karl Fischer method, Method A, using about 0.2 g of the substance; the water content is not less than 50 mg/g and not more than 75 mg/g.
pH value. pH of a 0.05 g/mL solution, 4.0-5.5.
Primary aromatic amines. Dissolve 0.10 g in 4 mL of hydrochloric acid (~70 g/L) TS using a 100-mL volumetric flask. Cool the solution in an ice-bath. In a test-tube, dissolve 50 mg of sodium nitrite R in 10 mL of water and cool the solution. To the flask in the ice-bath add half of the volume of the cooled sodium nitrite solution. Allow to stand for 10 minutes, lift the flask from the ice-bath, and add 1 g of urea R. Shake the flask frequently and when the evolution of gas has ceased (about 15 minutes), add 2.5 mL of sodium hydroxide (~80 g/L) TS in which 10 mg of thymol R has previously been dissolved. Add 5 mL of sodium hydroxide (~80 g/L) TS, allow to stand for 10 minutes, and dilute to volume. Prepare a blank as described above, but without the substance being examined. The test solution is not more intensely coloured than the blank solution when compared as described under 1.11.1 Colour of liquids.
Assay.
Dissolve 0.220 g in 50 mL of dehydrated ethanol R and add 5.0 mL of hydrochloric acid (0.01 mol/L) VS. Carry out a potentiometric titration using sodium hydroxide (0.1 mol/L) VS, as described under 2.6 Non-aqueous titration. Read the volume added between the two points of inflexion.
1 mL of 0.1 M sodium hydroxide (0.1 mol/L) VS is equivalent to 27.08 mg of C14H22N2O,HCl.
Additional requirements for Lidocaine hydrochloride for parenteral use
Complies with the monograph for "Parenteral preparations".
Bacterial endotoxins. Carry out the test as described under 3.4 Test for bacterial endotoxins; contains not more than 1.1 IU of endotoxin RS per mg.