Monographs: Pharmaceutical substances: Lindane (Lindanum)
Molecular formula. C6H6Cl6
Relative molecular mass. 290.8
Graphic formula.
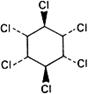
Chemical name. γ-1,2,3,4,5,6-Hexachlorocyclohexane; (1α,2α,3β,4α,5α,6β)-1,2,3,4,5,6-hexachlorocyclohexane; CAS Reg. No. 58-89-9.
Other names. Gamma benzene hexachloride; gammahexachlorcyclohexane.
Description. A white, crystalline powder; odour, slight.
Solubility. Practically insoluble in water; soluble in dehydrated ethanol R and ether R.
Category. Pediculicide; scabicide.
Storage. Lindane should be kept in a well-closed container.
Requirements
Definition. Lindane contains not less than 99.0% and not more than 100.5% of C6H6Cl6, calculated with reference to the anhydrous substance.
Identity tests
A. Carry out the examination as described under 1.7 Spectrophotometry in the infrared region. The infrared absorption spectrum is concordant with the spectrum obtained from lindane RS or with the reference spectrum of lindane.
B. To 1 mL of a 5.0 mg/mL solution add 3 mL of ethanol (~750 g/l) TS and 1 mL of potassium hydroxide/ethanol TS 1 and allow to stand for 10 minutes; the mixture yields reaction B, described under 2.1 General identification tests as characteristic of chlorides.
Congealing temperature. Not below 112.0°C.
Free chlorides. For the preparation of the test solution shake 1.2 g with 30 mL of water for 1 minute, and filter. To the filtrate add 10 mL of nitric acid (~130 g/l) TS and proceed as described under 2.2.1 Limit test for chlorides; the chloride content is not more than 0.2 mg/g.
Sulfated ash. Not more than 1.0 mg/g.
Water. Determine as described under 2.8 Determination of water by the Karl Fischer method, Method A, using about 1 g of the substance; the water content is not more than 5.0 mg/g.
Acidity or alkalinity. Boil 1.5 g with 30 mL of water for 1 minute and filter. To 10 mL of the filtrate add 2 drops of phenolphthalein/ethanol TS and titrate with carbonate-free sodium hydroxide (0.01 mol/l) VS; not more than 0.2 mL is required to obtain a pink colour. Add 0.4 mL of hydrochloric acid (0.01 mol/l) VS and 5 drops of methyl red/ethanol TS; the colour changes to orange.
Assay. To about 0.4 g, accurately weighed, add 25 mL of ethanol (~750 g/l) TS and warm on a water-bath until dissolved. Cool, add 10 mL of potassium hydroxide/ethanol (1 mol/l) VS, swirl gently, and allow to stand for 10 minutes. Dilute to 150 mL with water, neutralize with nitric acid (~130 g/l) TS, add 10 mL in excess of the acid, followed by 50 mL of silver nitrate (0.1 mol/l) VS. Filter, wash the residue with water, and titrate the combined filtrate and washings with ammonium thiocyanate (0.1 mol/l) VS, using ferric ammonium sulfate (45 g/l) TS as indicator. Repeat the operation without the substance being examined and make any necessary corrections. Each mL of silver nitrate (0.1 mol/l) VS is equivalent to 9.693 mg of C6H6Cl6.