Monographs: Pharmaceutical substances: Mannitol (Mannitolum)
Molecular formula. C6H14O6
Relative molecular mass. 182.2
Graphic formula.
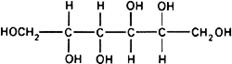
Chemical name. D-Mannitol; CAS Reg. No. 69-65-8.
Description. A white, crystalline powder; odourless.
Solubility. Freely soluble in water; very slightly soluble in ethanol (~750 g/l) TS: practically insoluble in ether R.
Category. Diuretic.
Storage. Mannitol should be kept in a well-closed container.
Additional information. Mannitol has a sweet taste.
Requirements
Definition. Mannitol contains not less than 98.0% and not more than 102.0% of C6H14O6, calculated with reference to the dried substance.
Identity tests
A. Transfer about 1.0 g, accurately weighed, to a 100-mL volumetric flask, and add 80 mL of ammonium molybdate (45 g/l) TS, previously filtered if necessary. Add sulfuric acid (~50 g/l) TS to volume and mix. Measure the optical rotation and calculate the specific rotation as described under 1.4 Determination of optical rotation and specific rotation;
= +137° to +145°.
B. To 0.5 g add 2.5 mL of acetyl chloride R, then add cautiously 0.5 mL of pyridine R. Warm the mixture until it becomes turbid, cool in ice, and collect the precipitate on a sintered-glass filter. Recrystallize the precipitate several times from ether R and dry at 60°C for 1 hour; melting temperature, about 123°C (mannitol hexaacetate).
Melting range. 165-169°C.
Heavy metals. Use 1.0 g for the preparation of the test solution as described under 2.2.3 Limit test for heavy metals, Procedure 1; determine the heavy metals content according to method A; not more than 10 μg/g.
Arsenic. Use a solution of 5.0 g in 35 mL of water and proceed as described under 2.2.5 Limit test for arsenic; the arsenic content is not more than 2 μg/g.
Chlorides. Dissolve 2.5 g in a mixture of 2 mL of nitric acid (~130 g/l) TS and 30 mL of water and proceed as described under 2.2.1 Limit test for chlorides; the chloride content is not more than 0.1 mg/g.
Sulfates. Dissolve 5.0 g in 40 mL of water and proceed as described under 2.2.2 Limit test for sulfates; the sulfate content is not more than 0.1 mg/g.
Clarity and colour of solution. A solution of 1.0 g in 10 mL of carbon-dioxide-free water R is clear and colourless.
Sulfated ash. Not more than 1.0 mg/g.
Loss on drying. Dry to constant weight at 105°C; it loses not more than 5.0 mg/g.
Acidity. Dissolve 5.0 g in 50 mL of carbon-dioxide-free water R and titrate with carbonate-free sodium hydroxide (0.02 mol/l) VS, phenolphthalein/ethanol TS being used as indicator; not more than 0.3 mL is required to obtain the midpoint of the indicator (pink).
Sorbitol. Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography, using silica gel R1 as the coating substance and a mixture of 85 volumes of 2-propanol R and 15 volumes of a 2 g/l solution of boric acid R as the mobile phase. Prepare a solution of 1.0 g of finely powdered test substance in 10 mL of ethanol (~750 g/l) TS, shake for 30 minutes and filter (solution A). Apply separately to the plate 1 μl of test solution A and 2 μl of a 1.0 mg/mL solution of sorbitol R in water (B).
Develop the plate at room temperature, the process taking up to 5 hours. After removing the plate from the chromatographic chamber, allow it to dry at 110°C for 5 minutes, cool, and spray with a 1 g/l solution of potassium permanganate R in sulfuric acid (0.5 mol/l) VS. Heat the plate at 110°C until brown spots appear and examine the chromatogram in daylight. The spot obtained with solution B is more intense than any spot, corresponding in position and appearance, obtained with solution A.
Assay. Dissolve about 0.4 g, accurately weighed, in sufficient water to produce 100 mL. Transfer 10 mL to a stoppered flask, add 20.0 mL of a 21.4 g/l solution of sodium metaperiodate R and 2 mL of sulfuric acid (~100 g/l) TS and heat on a water-bath for 15 minutes. Cool, add 3 g of sodium hydrogen carbonate R, 25 mL of sodium arsenite (0.05 mol/l) VS, and 5 mL of a 200 g/l solution of potassium iodide R; allow to stand for 15 minutes, and titrate with iodine (0.05 mol/l) VS until the first trace of yellow colour appears. Repeat the procedure without the test substance and determine the difference in volume of iodine (0.05 mol/l) VS required for the titration. Each mL of iodine (0.05 mol/l) VS is equivalent to 1.822 mg of C6H14O6.
Additional requirements for Mannitol for parenteral use
Complies with the monograph for "Parenteral preparations".
Bacterial endotoxins. Carry out the test as described under 3.4 Test for bacterial endotoxins; contains not more than 4 IU of endotoxin RS per g for dosage forms with a concentration of less than 100 g/l of mannitol, and a limit of not more than 2.5 IU of endotoxin RS per g for dosage forms with a concentration of 100 g/l or more of mannitol.