Monographs: Pharmaceutical substances: Medroxyprogesterone acetate (Medroxyprogesteroni acetas)
Molecular formula. C24H34O4
Relative molecular mass. 386.5
Graphic formula
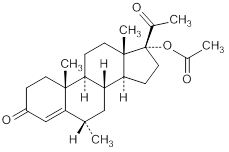
Chemical name. 6α-methyl-3-20-dioxopregn-4-en-17-yl acetate, (6α)-17-(acetyloxy)-6-methylpregn-4-ene-3,20-dione, 17-hydroxy-6α-methylprogesterone acetate; CAS Reg. No. 71-58-9.
Description. A white or almost white, crystalline powder.
Solubility. Practically insoluble in water; soluble in acetone R and dioxan R; slightly soluble in ethanol (~750 g/L) TS and methanol R .
Category. Progestogen.
Storage. Medroxyprogesterone acetate should be kept in a tight container, protected from light.
Requirements
Definition. Medroxyprogesterone acetate contains not less than 97.0% and not more than 103.0% of C24H34O4, calculated with reference to the dried substance.
Identity tests
- Either test A or tests B and C may be applied.
A. Carry out the examination as described under 1.7 Spectrophotometry in the infrared region. The infrared absorption spectrum is concordant with the spectrum obtained from medroxyprogesterone acetate RS or with the reference spectrum of medroxyprogesterone acetate.
B. Carry out test B.1 or, where UV detection is not available, test B.2.
B.1 Carry out the test as described under 1.14.1. Thin-layer chromatography using silica R5 as the coating substance and a mixture of 10 volumes of dichloromethane R and 1 volume of ethyl acetate R as the mobile phase. Apply separately to the plate 10 μL of each of the following two solutions in dichloromethane R. For solution (A) use 2.5 mg of the test substance per mL. For solution (B) use 2.5 mg of medroxyprogesterone acetate RS per mL. After removing the plate from the chromatographic chamber heat it at 120 °C for 30 minutes, spray with 4-toluenesulfonic acid/ethanol TS and heat further at 120 °C for 10 minutes. Allow the plate to cool and examine the chromatogram in ultraviolet light (365 nm).
The principal spot obtained with solution (A) corresponds in position, appearance and intensity to that obtained with solution (B).
B.2 Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography using the conditions described under test B.1, but spray the plate with a mixture of equal volumes of sulfuric acid R and ethanol (~750 g/L) TS and heat further at 120 °C for 10 minutes. Allow the plate to cool and examine the chromatogram in daylight.
The principal spot obtained with solution (A) corresponds in position, appearance and intensity to that obtained with solution (B).
C. Use 20 mg; it yields the reaction described under 2.1 General identification tests as characteristic of acetylated substances.
Specific optical rotation. Use a 10 mg/mL solution in acetone R;  = +47° to +53° with reference to the dried substance.
= +47° to +53° with reference to the dried substance.
Sulfated ash. Not more than 1.0 mg/g.
Loss on drying. Dry at 105 °C for 3 hours; it loses not more than 10 mg/g.
Impurity F
Carry out the test as described under 1.14.1 Chromatography, High-performance liquid chromatography using a stainless steel column (10 cm × 4.6 mm) packed with end-capped particles of silica gel, the surface of which has been modified with chemically-bonded octadecylsilyl groups (3 µm).
As the mobile phase use a solution prepared as follows: mix 44 volumes of water R and 56 volumes of acetonitrile R.
For solution (1) dissolve 20 mg of the test substance in 5.0 mL of acetonitrile R and dilute to 10.0 mL with water R. For solution (2) dilute 0.5 volume of solution (1) to 100 volumes with the mobile phase. For solution (3) use a solution containing about 0.2 mg of medroxyprogesterone acetate RS and 0.01 mg of medroxyprogesterone acetate impurity F RS per mL mobile phase.
Operate with a flow rate of 1.0 mL per minute. As a detector use an ultraviolet spectrophotometer set at a wavelength of 200 nm.
Inject 25 µL of solution (1), (2) and (3). In the chromatogram obtained with solution (3) impurity F is eluted at a relative retention of about 1.8 with reference to medroxyprogesterone acetate (retention time about 8 minutes).
In the chromatogram obtained with solution (1) the area of any peak corresponding to impurity F, when multiplied by a correction factor of 1.8, is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (0.5%).
Related substances
Prepare fresh solutions and perform the tests without delay.
Carry out the test as described under 1.14.1 Chromatography, High-performance liquid chromatography using a stainless steel column (25 cm × 3.0 mm) packed with end-capped particles of silica gel, the surface of which has been modified with chemically-bonded octadecylsilyl groups (5 µm).
Maintain the column temperature at 60 °C.
As the mobile phase, use a solution prepared as follows: mix 12 volumes of tetrahydrofuran R, 23 volumes of acetonitrile R and 65 volumes of water R.
Prepare the following solutions in a solvent prepared by mixing equal volumes of acetonitrile R and water R.
For solution (1) dissolve 20 mg of the test substance and dilute to 10.0 mL. For solution (2) dilute 1.0 mL of solution (1) to 100.0 mL. For solution (3) dilute 1.0 mL of solution (2) to 10.0 mL. For solution (4) dissolve 4 mg of medroxyprogesterone acetate for system suitability RS (containing medroxyprogesterone acetate and the impurities A, B, C, D, E, G and I) and dilute to 2.0 mL.
Operate with a flow rate of 0.9 mL per minute. As a detector use an ultraviolet spectrophotometer set at a wavelength of 254 nm.
Inject separately 20 µL of solution (1), (2), (3) and (4). Record the chromatogram for about twice the retention time of medroxyprogesterone acetate in solution (2).
Use the chromatogram supplied with medroxyprogesterone acetate for system suitability RS and the chromatogram obtained with solution (4) to identify the peaks due to impurities A, B, C, D, E, G and I. The impurities are eluted at the following relative retention with reference to the peak of medroxyprogesterone acetate (retention time about 27 minutes): impurity A about 0.3; impurity I about 0.5; impurity H about 0.65; impurity B about 0.7; impurity C about 0.8; impurity G about 0.85; impurity D about 0.9; impurity E about 0.95.
The test is not valid unless in the chromatogram obtained with solution (4) the peak-to-valley ratio (Hp/Hv) is at least 2.5, where Hp is the height above the baseline of the peak due to impurity E and Hv is the height above the baseline of the lowest point of the curve separating this peak from the peak due to medroxyprogesterone acetate.
In the chromatogram obtained with solution (1):
- the area of any peak corresponding to impurity D is not greater than the area of the principal peak obtained with solution (2) (1.0%);
- the area of any peak corresponding to impurity B is not greater than 0.7 times the area of the principal peak obtained with solution (2) (0.7%);
- the area of any peak corresponding to impurity A, when multiplied by a correction factor of 1.5, is not greater than 3 times of the area of the principal peak obtained with solution (3) (0.3%);
- the area of any peak corresponding to impurity G, when multiplied by a correction factor of 2.6, is not greater than 2 times of the area of the principal peak obtained with solution (3) (0.2%);
- the area of any peak corresponding to impurity C, E or I is not greater than 2 times the area of the principal peak obtained with solution (3) (0.2%);
- the area of any other impurity peak is not greater than the area of the principal peak obtained with solution (3) (0.1%);
- the sum of the corrected areas of any peak corresponding to impurity A or impurity G and the areas of all other impurity peaks is not greater than 1.5 times the area of the principal peak obtained with solution (2) (1.5%). Disregard any peak with an area less than 0.5 times the area of the principal peak obtained with solution (3) (0.05%).
Assay
Dissolve about 0.100 g, accurately weighed, in ethanol (~750g/L) TS to produce 100.0 mL; dilute 1.0 mL of this solution to 100 mL with the same solvent.
Measure the absorbance of the diluted solution in a 1 cm layer at the maximum at about 241 nm and calculate the content of C24H34O4 using the absorptivity value of 42.6 (  = 426)
= 426)
Impurities
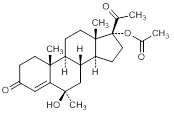
A. 6β-hydroxy-6α-methyl-3-20-dioxopregn-4-en-17-yl acetate (6-hydroxymedroxyprogesterone-17-acetate)
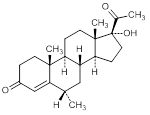
B. 17-hydroxy-6α-methylpregn-4-ene-3-20-dione (medroxyprogesterone)
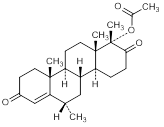
C. 6α,17aβ-dimethyl-3-17-dioxo-D-homoandrost-4-en-17aα-yl acetate,
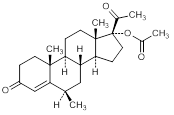
D. 6β-methyl-3-20-dioxopregn-4-en-17-yl acetate (6-epi-medroxyprogesterone acetate)
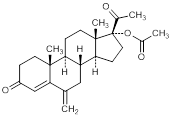
E. 6-methylidene-3-20-dioxopregn-4-en-17-yl acetate (6-methylidenehydroxyprogesterone acetate)
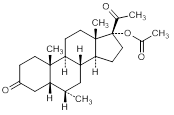
F. 6α-methyl-3-20-dioxo-5β-pregnan-17-yl acetate (4,5β-dihydromedroxyprogesterone acetate)
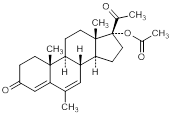
G. 6-methyl-3-20-dioxopregna-4,6-dien-17-yl acetate (megestrol acetate)
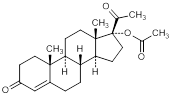
H. 3-20-dioxopregn-4-en-17-yl acetate (hydroxyprogesterone acetate)
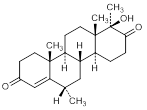
I. 17aβ-hydroxy-6α,17aα-dimethyl-D-homoandrost-4-ene-3-17-dione