Monographs: Pharmaceutical substances: Methotrexate (Methotrexatum)
Molecular formula. C20H22N8O5
Relative molecular mass. 454.4
Graphic formula.
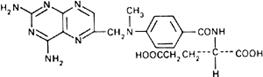
Chemical name. (+)-N-[p-[[(2,4-Diamino-6-pteridinyl)methyl]methylamino]benzoyl]-L-glutamic acid; N-[4-[[(2,4-diamino-6-pteridinyl)methyl]methylamino]benzoyl]-L-glutamic acid; CAS Reg. No. 59-05-2.
Description. A yellow to orange, crystalline powder.
Solubility. Practically insoluble in water, ethanol (~750 g/l) TS, dichloroethane R, and ether R; very soluble in diluted solutions of alkali hydroxides and carbonates.
Category. Cytotoxic drug.
Storage. Methotrexate should be kept in a tightly closed container, protected from light.
Additional information. Methotrexate is gradually affected by light. CAUTION: Methotrexate must be handled with care, avoiding contact with the skin and inhalation of airborne particles.
Requirements
Definition. Methotrexate contains not less than 96.0% and not more than 102.0% of C20H22N8O5, calculated with reference to the anhydrous substance.
Identity tests
A. Carry out the examination as described under 1.7 Spectrophotometry in the infrared region. The infrared absorption spectrum is concordant with the spectrum obtained from methotrexate RS or with the reference spectrum of methotrexate.
B. The absorption spectrum of a 10.0 μg/mL solution in sodium hydroxide (0.1 mol/l) VS, when observed between 230 nm and 380 nm, exhibits 3 maxima at about 258 nm, 303 nm, and 371 nm. The ratio of the absorbance at 303 nm to that at 371 nm is between 2.8 and 3.3.
Specific optical rotation. Dissolve 0.25 g in 12 mL of sodium carbonate (10 g/l) TS, dilute with water to 25 mL, and calculate the result with reference to the anhydrous substance;  = +19° to +24°.
= +19° to +24°.
Sulfated ash. Not more than 1.0 mg/g.
Water. Determine as described under 2.8 Determination of water by the Karl Fischer method, Method A, using about 0.5 g of the substance; the water content is not more than 120 mg/g.
Assay. Carry out the test as described under 1.14.1 Chromatography, High-performance liquid chromatography, using a column 10 cm long and 6 mm in internal diameter packed with silica gel, 5 μm in diameter, the surface of which has been modified with chemically bonded octadecylsilyl groups.
As the mobile phase, use a mixture of 8 volumes of acetonitrile R with 92 volumes of phosphate/citrate buffer pH 6.0, TS.
Prepare the following solutions in the above-mentioned mobile phase containing (A) 0.10 mg of the test substance per mL, (B) 0.10 mg of methotrexate RS per mL, and (C) 0.10 mg of methotrexate RS and 0.10 mg of folic acid RS per mL for the system suitability test.
Operate at room temperature with a flow rate of about 1.4 mL per minute. As a detector use an ultraviolet spectrophotometer at a wavelength of about 303 nm, fitted with a low-volume flow cell (10 μl is suitable), and a suitable recorder.
Make 6 replicate injections of solution C, each of 20 μl. The resolution factor between methotrexate and folic acid should be not less than 5.0, with a relative standard deviation for the methotrexate peak of not more than 2.5% (adjust the flow rate and the ratio of the mobile phase if it does not conform).
Inject 20 μl of each of solutions A and B. Measure the peak responses and calculate the content in % of C20H22N8O5 using the following formula: 100(A1M2T)/(A2M1), in which A1 and A2 are the peak responses of the test substance and the reference substance, respectively, and M1 and M2 are the concentrations of the test solution and the reference solution, respectively, and T corresponds to the degree of purity of methotrexate RS.
Additional requirement for Methotrexate for parenteral use
Complies with the monograph for "Parenteral preparations".