Monographs: Pharmaceutical substances: Metronidazole (Metronidazolum)
Molecular formula. C6H9N3O3
Relative molecular mass. 171.2
Graphic formula.
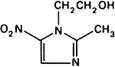
Chemical name. 2-Methyl-5-nitroimidazole-1-ethanol; 2-methyl-5-nitro-1H-imidazole-1-ethanol; CAS Reg. No. 443-48-1.
Description. A white or pale yellow, crystalline powder; odourless or almost odourless.
Solubility. Sparingly soluble in water; slightly soluble in ethanol (~750 g/l) TS and ether R.
Category. Antitrichomonal; antiamoebic.
Storage. Metronidazole should be kept in a well-closed container, protected from light.
Additional information. Metronidazole is stable in air, but darkens on exposure to light.
Requirements
Definition. Metronidazole contains not less than 99.0% and not more than 101.0% of C6H9N3O3, calculated with reference to the dried substance.
Identity tests
A. Carry out the examination as described under 1.7 Spectrophotometry in the infrared region. The infrared absorption spectrum is concordant with the spectrum obtained from metronidazole RS or with the reference spectrum of metronidazole.
B. To 10 mg add 10 mg of zinc R powder, 1 mL of water, and 0.25 mL of hydrochloric acid (~250 g/l) TS and heat in a water-bath for 5 minutes; cool in ice, add 0.5 mL of sodium nitrite (100 g/l) TS, and remove the excess nitrite with sufficient sulfamic acid (50 g/l) TS. Add 0.5 mL of the resulting solution to a mixture of 0.5 mL of 2-naphthol TS1 and 2 mL of sodium hydroxide (~80 g/l) TS; an orange-red colour is produced.
Melting range. 159-163°C.
Sulfated ash. Not more than 1.0 mg/g.
Loss on drying. Dry to constant weight at 105°C; it loses not more than 5.0 mg/g.
Related substances. Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography, using silica gel R2 as the coating substance and a mixture of 9 volumes of chloroform R and 1 volume of diethylamine R as the mobile phase. Apply separately to the plate 5 μl of each of 2 solutions in acetone R containing (A) 20 mg of the test substance per mL (warm slightly if necessary to dissolve the substance) and (B) 0.10 mg of the test substance per mL. After removing the plate from the chromatographic chamber, allow it to dry in air, and examine the chromatogram in ultraviolet light (254 nm). Any spot obtained with solution A, other than the principal spot, is not more intense than that obtained with solution B.
Assay. Dissolve about 0.35 g, accurately weighed, in 30 mL of glacial acetic acid R1, add 3 drops of 1-naphtholbenzein/acetic acid TS as indicator and titrate with perchloric acid (0.1 mol/l) VS, as described under 2.6 Non-aqueous titration, Method A. Each mL of perchloric acid (0.1 mol/l) VS is equivalent to 17.12 mg of C6H9N3O3.
Additional requirements for Metronidazole for parenteral use
Complies with the monograph for "Parenteral preparations".
Bacterial endotoxins. Carry out the test as described under 3.4 Test for bacterial endotoxins; contains not more than 0.35 IU of endotoxin RS per mg.