Monographs: Pharmaceutical substances: Metronidazole benzoate (Metronidazoli benzoas)
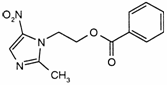
C13H13N3O4
Relative molecular mass. 275.3
Chemical name. 2-(2-Methyl-5-nitro-1H-imidazol-1-yl)ethyl benzoate; 2-methyl-5-nitro-1H-imidazole-1-ethanol benzoate; CAS Reg. No. 13182-89-3.
Description. A white or slightly yellowish, crystalline powder.
Solubility. Practically insoluble in water; freely soluble in dichloromethane R; soluble in acetone R; slightly soluble in ethanol (~750 g/l) TS; very slightly soluble in ether R.
Category. Anti-infective drug.
Storage. Metronidazole benzoate should be kept in a well-closed container, protected from light.
Requirements
Metronidazole benzoate contains not less than 98.5% and not more than 101.0% of C13H13N3O4, calculated with reference to the dried substance.
Identity tests
• Either tests A and D or tests B, C, and D may be applied.
A. Carry out the examination as described under 1.7 Spectrophotometry in the infrared region. The infrared absorption spectrum is concordant with the spectrum obtained from metronidazole benzoate RS or with the reference spectrum of metronidazole benzoate.
B. See the test described below under "Related substances". The principal spot obtained with solution B corresponds in position, appearance, and intensity with that obtained with solution C.
C. To about 10 mg add 10 mg of zinc R powder, 1 mL of water, and about 0.3 mL of hydrochloric acid (~420 g/l) TS. Heat on a water-bath for 5 minutes and cool. The solution yields the reaction described for the identification of primary aromatic amines under 2.1 General identification tests, producing a red precipitate.
D. Melting temperature, about 101 °C.
Heavy metals. Use 1.0 g for the preparation of the test solution as described under 2.2.3 Limit test for heavy metals, Procedure 3; determine the heavy metals content according to Method A; not more than 20 μg/g.
Sulfated ash. Not more than 1.0 mg/g.
Loss on drying. Dry at 80 °C for 3 hours; it loses not more than 5.0 mg/g.
pH value. pH of a 20 mg/mL suspension in carbon-dioxide-free water R, 5.0-7.0.
Related substances. Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography, using silica gel R2 as the coating substance. Heat to activate the plate at 110 °C for 1 hour and cool before use. As the mobile phase, use ethyl acetate R. Apply separately to the plate 10 μl of each of 8 solutions in acetone R containing (A) 20 mg of Metronidazole benzoate per mL, (B) 2.0 mg of Metronidazole benzoate per mL, (C) 2.0 mg of metronidazole benzoate RS per mL, (D) 0.10 mg of Metronidazole benzoate per mL, (E) 0.040 mg of Metronidazole benzoate per mL, (F) 0.10 mg of metronidazole RS per mL, (G) 0.10 mg of 2-methyl-5-nitroimidazole R per mL, and for solution (H) dissolve 10 mg of metronidazole RS and 10 mg of 2-methyl-5-nitroimidazole R in sufficient acetone R to produce 50 mL. After removing the plate from the chromatographic chamber, allow it to dry in air, and examine the chromatogram in ultraviolet light (254 nm).
Any spot corresponding to metronidazole or to 2-methyl-5-nitroimidazole obtained with solution A is not more intense than the corresponding spot obtained with solutions F and G (0.5%). Any spot obtained with solution A, other than the principal spot and the spots corresponding to metronidazole and to 2-methyl-5-nitroimidazole, is not more intense than that obtained with solution D (0.5%), and not more than one such spot is more intense than that obtained with solution E (0.2%). The test is not valid unless the chromatogram obtained with solution H shows two clearly separated principal spots.
Assay. Dissolve about 0.25 g, accurately weighed, in 50 mL of glacial acetic acid R1, and titrate with perchloric acid (0.1 mol/l) VS as described under 2.6 Non-aqueous titration, Method A, determining the end-point potentiometrically.
Each mL of perchloric acid (0.1 mol/l) VS is equivalent to 27.53 mg of C13H13N3O4.