Monographs: Pharmaceutical substances: Molnupiravir (Molnupiravirum)
Molecular formula. C13H19N3O7
Relative molecular mass. 329.31
Graphic formula.
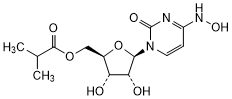
Chemical name. {(2R,3S,4R,5R)-3,4-Dihydroxy-5-[4-(hydroxyamino)-2-oxopyrimidin-1(2H)-yl]oxolan-2-yl}methyl 2-methylpropanoate or {(2R,3S,4R,5R)-3,4-Dihydroxy-5-[(4Z)-4-(hydroxyimino)-2-oxo-3,4-dihydropyrimidin-1(2H)-yl]oxolan-2-yl}methyl 2-methylpropanoate or N4-Hydroxycytidine 5'-(2-methylpropanoate) (IUPAC); Uridine, 4-oxime, 5'-(2-methylpropanoate), (4Z)- (CAS).
CAS Registry Number. 2492423-29-5
Description. A white to off-white powder.
Solubility. It is freely soluble in methanol R and dimethyl sulfoxide R, soluble in water R and, sparingly soluble in 2-propanol R, slightly soluble in dehydrated ethanol R, ethyl acetate R and acetonitrile R. It is practically insoluble in dichloromethane R, n-heptane R, and n-hexane R.
Category. Antiviral.
Storage. Molnupiravir should be kept in tightly closed containers, protected from moisture.
Additional information. Molnupiravir is slightly hygroscopic and exhibits polymorphism.
Requirements
Manufacture. The production method is validated to demonstrate that the substance hydroxylamine is adequately controlled in the final product. If tested with a suitable method, the substance would comply with a hydroxylamine limit of not more than 14 ppm.
Definition. Molnupiravir contains not less than 97.0% and not more than 102.0%of C13H19N3O7, calculated with reference to the anhydrous substance.
Identity tests
-
Either test A or any two of tests B, C and D may be applied.
-
Carry out the test as described under 1.7 Spectrophotometry in the infrared region. The infrared absorption spectrum is concordant with the spectrum obtained from molnupiravir RS or with the reference spectrum of molnupiravir.
If the spectra thus obtained are not concordant, repeat the test using the residues obtained by separately dissolving the test substance and molnupiravir RS in a small amount of methanol R and evaporating to dryness. The infrared absorption spectrum is concordant with the spectrum obtained from molnupiravir RS.
-
Carry out the test as described under 1.14.1 Chromatography, High-performance liquid chromatography, using the conditions given under "Assay". The retention time of the principal peak in the chromatogram obtained with solution (1) corresponds to the retention time of the peak due to molnupiravir in the chromatogram obtained with solution (2).
-
Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography, using silica gel R6 as the coating substance and a freshly prepared mixture of ethyl acetate R, methanol R and glacial acetic acid R (90:9:1 V/V/V) as the mobile phase. Apply separately to the plate 2 µL of each of the following two solutions in methanol R, containing (A) 1 mg per mL of the test substance and (B) 1 mg per mL of molnupiravir RS. After removing the plate from the chromatographic chamber, allow it to dry in air or in a current of air. Examine the chromatogram under ultraviolet light (254 nm).
The principal spot in the chromatogram obtained with solution (A) corresponds in position, appearance and intensity with the spot due to molnupiravir in the chromatogram obtained with solution (B).
-
The absorption spectrum (1.6) of a 0.03 mg per mL solution of the test substance in methanol R, when observed between 200 nm and 400 nm, exhibits two maxima at about 235 nm and 275 nm.
Alternatively, in combination with identity test B, where a diode-array detector is available, record the UV spectrum of the principal peak in the chromatograms with a diode array detector in the range of 200 nm to 400 nm. The UV spectrum of the principal peak in the chromatogram obtained with solution (1) corresponds to the UV spectrum of the peak due to molnupiravir in the chromatogram obtained with solution (2).
Specific optical rotation (1.4). Use a 10.0 mg per mL solution of the test substance in methanol R and calculate with reference to the anhydrous substance. The specific optical rotation 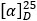 is between -11.0 to -6.0.
is between -11.0 to -6.0.
Water. Determine as described under 2.8 Determination of water by the Karl Fischer method, Method A. Use 0.300 g of the test substance. The water content is not more than 10 mg/g.
Heavy metals. Use 0.300 g for the preparation of the test solution as described under 2.2.3 Limit test for heavy metals, Procedure 5. Determine the heavy metals content according to Method C; not more than 10 μg/g.
Related substances. Carry out the test as described under 1.14.1 Chromatography, High-performance liquid chromatography, using a stainless-steel column (4.6 mm x 25 cm) packed with particles of silica gel, the surface of which has been modified with chemically bonded phenyl groups (5 µm).
Use the following conditions for gradient elution:
-
mobile phase A: pH 2.3 buffer solution;
-
mobile phase B: mixture of 20 volumes of water R and 80 volumes of the solvent mixture.
Prepare the pH 2.3 buffer solutionby dissolving 3.4 g of potassium dihydrogen phosphate R in water R and diluting to 1000 mL with the same solvent. Carefully adjust the pH to 2.3 with phosphoric acid (~105 g/L) TS.
Prepare the solvent mixture by mixing 30 volumes of methanol R and 70 volumes of acetonitrile for chromatography R.
|
Time (minutes) |
Mobile phase A (% V/V) |
Mobile phase B (% V/V) |
Comments |
|
0–5 |
100 |
0 |
Isocratic |
|
5–20 |
100 to 80 |
0 to 20 |
Linear gradient |
|
20–40 |
80 to 75 |
20 to 25 |
Linear gradient |
|
40–55 |
75 to 40 |
25 to 60 |
Linear gradient |
|
55–65 |
40 to 0 |
60 to 100 |
Linear gradient |
|
65–73 |
0 |
100 |
Isocratic |
|
73–74 |
0 to 100 |
100 to 0 |
Return to initial composition |
|
74–85 |
100 |
0 |
Re-equilibration |
Operate with a flow rate of 0.9 mL per minute. As a detector, use an ultraviolet spectrophotometer set at a wavelength of 230 nm, for impurities F and L at 260 nm and for impurity G at 210 nm. Maintain the column temperature at 25 °C.
Prepare the following solutions freshly and perform the analysis without delay. Use water R as a diluent. For solution (1), transfer 120 mg of the test substance into a 100 mL volumetric flask. Add 60 mL of the diluent, allow to cool to room temperature and make up to volume. For solution (2), dilute 1.0 mL of solution (1) to 100.0 mL. Dilute 1.0 mL of this solution to 10.0 mL. For solution (3), dilute 5.0 mL of solution (2) to 10.0 mL. For solution (4), dissolve 12 mg of molnupiravir RS(containing molnupiravir and the impurities A and I) in 10 mL.
Inject 20 µL each of solutions (1), (2), (3) and (4).
Use the chromatogram obtained with solution (4) and the chromatogram supplied with molnupiravir RS to identify impurity A and I.
The impurities are eluted, if present, at the following relative retentions with reference to molnupiravir (retention time about 23 minutes): impurity D about 0.19; impurity A about 0.23; impurity E about 0.45; impurity L about 0.82; impurity I about 1.03, impurity F about 1.14; impurity G about 1.70 and 1.72, impurity B about 1.83 and impurity H about 2.04.
The test is not valid unless, in the chromatogram obtained with solution (4), the peak-to-valley ratio (Hp/Hv) is at least 3.0, where Hp is the height above the baseline of the peak due to impurity I and Hv is the height above the baseline of the lowest point of the curve separating the peak due to molnupiravir from the peak due to impurity I. Also, the test is not valid unless, in the chromatogram obtained with solution (3), the peak due to molnupiravir is obtained with a signal-to-noise ratio of at least 10.
In the chromatogram obtained with solution (1):
-
the area of any peak corresponding to impurity A is not greater than 8 times the area of the peak due to molnupiravir in the chromatogram obtained with solution (2) (0.80 %);
-
the area of any peak corresponding to impurity B is not greater than 2.2 times the area of the peak due to molnupiravir in the chromatogram obtained with solution (2) (0.22 %);
-
the area of any peak corresponding to impurity I is not greater than the area of the peak due to molnupiravir in the chromatogram obtained with solution (2) (0.10 %);
-
the area of any other impurity peakis not greater than0.6 times the area of the peak due to molnupiravir in the chromatogram obtained with solution (2) (0.06 %);
-
the sum of the areas of any peaks corresponding to impurity G, recorded at 210 nm, is not greater than 0.6 times the area of the peak due to molnupiravir in the chromatogram obtained with solution (2), recorded at 210 nm (0.06 %);
-
the area of any peak corresponding to impurity F, recorded at 260 nm, when multiplied with a correction factor of 0.7, is not greater than 0.6 times the area of the peak due to molnupiravir in the chromatogram obtained with solution (2), recorded at 260 nm (0.06 %);
-
the area of any peak corresponding to impurity L, recorded at 260 nm is not greater than 0.6 times the area of the peak due to molnupiravir in the chromatogram obtained with solution (2), recorded at 260 nm (0.06 %).
-
Determine the sum of the areas of all impurity peaks recorded at 230 nm, excluding the areas of the peaks due to impurities G, F, and L. Disregard any peaks with an area of less than the area of the peak due to molnupiravir in the chromatogram obtained with solution (3), recorded at 230 nm (0.05%).Calculate the percentage content of all impurities using the area of the peak due to molnupiravir in the chromatogram obtained with solution (2), recorded at 230 nm, as a reference.
-
Determine the sum of the area of any peak corresponding to impurity L and the corrected area of any peak corresponding to impurity F, recorded at 260 nm, and calculate their percentage content using the area of the peak due to molnupiravir in the chromatogram obtained with solution (2), recorded at 260 nm, as a reference. Disregard any peaks due to impurities L or G with an area of less than the area of the peak due to molnupiravir in the chromatogram obtained with solution (3), recorded at 260 nm (0.05%).
-
Determine the area of any peak corresponding to impurity G, recorded at 210 nm, and calculate its percentage content using the area of the peak due to molnupiravir in the chromatogram obtained with solution (2), recorded at 210 nm, as a reference. Disregard any peak due to impurity G with an area of less than the area of the peak due to molnupiravir in the chromatogram obtained with solution (3), recorded at 210 nm (0.05%).
-
The sum of the percentage content of all impurities, recorded at 230 nm, and the percentage contents of the impurities F, L and G, recorded at 260 nm and 210 nm respectively, is not greater than 1.0 %.
Assay. Carry out the test as described under 1.14.1 Chromatography, High-performance liquid chromatography, using a stainless steel column (4.6 mm x 15 cm) packed with end-capped particles of silica gel, the surface of which has been modified with chemically-bonded phenyl groups (2.6 µm).
Use the following conditions for gradient elution:
-
mobile phase A: ammonium dihydrogen phosphate solution;
-
mobile phase B: acetonitrile for chromatography R.
Prepare the ammonium dihydrogen phosphate solution by dissolving 5.75 g of ammonium dihydrogen phosphate R in water R and diluting to 1000 mL with the same solvent.
|
Time (minutes) |
Mobile phase A (% V/V) |
Mobile phase B (% V/V) |
Comments |
|
0–15 |
90 |
10 |
Isocratic |
|
15–16 |
90 to 35 |
10 to 65 |
Linear gradient |
|
16–22 |
35 |
65 |
Isocratic |
|
22–23 |
35 to 90 |
65 to 10 |
Return to initial composition |
|
23–30 |
90 |
10 |
Re-equilibration |
Operate with a flow rate of 0.7 mL per minute. As a detector, use an ultraviolet spectrophotometer set at a wavelength of 260 nm. Maintain the column temperature at 40 °C.
Prepare a buffer solution pH 4.75 by dissolving 3.08 g of ammonium acetate R in water R and diluting to 2000 mL with the same solvent. Adjust the pH to a value between 4.70 and 4.80 using glacial acetic acid R.
Prepare as the diluent, a mixture of 90 volumes of buffer solution pH 4.75 and 10 volumes of methanol R.
Prepare the following solutions. For solution (1), transfer 60.0 mg of the test substance into a 50 mL volumetric flask. Add about 30 mL of the diluent, sonicate to dissolve, allow to cool to room temperature, make up to volume, mix and filter. Dilute 5.0 mL of this solution to 50.0 mL. For solution (2), weigh 60.0 mg of molnupiravir RS into a 50 mL volumetric flask. Add 30 mL of the diluent, sonicate to dissolve and make up to volume. Dilute 5.0 mL of this solution to 50.0 mL.
Inject 10 µL each of solutions (1) and (2) and record the chromatograms.
Measure the areas of the peaks corresponding to molnupiravir obtained in the chromatograms of solutions (1) and (2) and calculate the percentage content of Molnupiravir (C13H19N3O7) in the sample using the declared content of C13H19N3O7 in molnupiravir RS.
Impurities
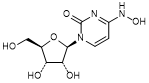
A. N4-Hydroxycytidine or 1-[(2R,3R,4S,5R)-3,4-Dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-4-(hydroxyamino)pyrimidin-2(1H)-one (synthesis related impurity, degradation product),
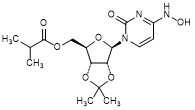
B. {(3aR,4R,6R,6aR)-6-[4-(Hydroxyamino)-2-oxopyrimidin-1(2H)-yl]-2,2-dimethyltetrahydro-2H-furo[3,4-d][1,3]dioxol-4-yl}methyl 2-methylpropanoate or N4-Hydroxy-O2′,O3′-propan-2-ylidenecytidin-5′-yl 2-methylpropanoate(dimethyl dioxol impurity, molnupiravir acetonide)(synthesis related impurity),
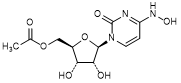
C. {(2R,3S,4R,5R)-3,4-Dihydroxy-5-[4-(hydroxyamino)-2-oxopyrimidin-1(2H)-yl]oxolan-2-yl}methyl acetate or N4-Hydroxycytidine 5′-O- acetate (molnupiravir acetyl analog) (synthesis related impurity),
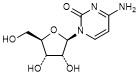
D. Cytidine or 4-Amino-1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2(1H)-one (synthesis related impurity),
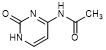
E. N4-Acetylcytosine or N-{1-[(2R,3R,4S,5R)-3,4-Dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-2-oxo-1,2-dihydropyrimidin-4-yl}acetamide (synthesis related impurity),
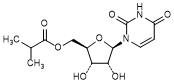
F. [(2R,3S,4R,5R)-5-(2,4-Dioxo-3,4-dihydropyrimidin-1(2H)-yl)-3,4-dihydroxyoxolan-2-yl]methyl 2-methylpropanoate or uridine 5′-O-(2-methylpropanoate) (synthesis related impurity, degradation product),
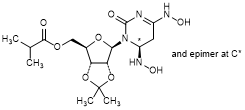
G. {(3aR,4R,6R,6aR)-6-[(6RS)-4,6-Bis(hydroxyamino)-2-oxo-5,6-dihydropyrimidin-1(2H)-yl]-2,2-dimethyltetrahydro-2H-furo[3,4-d][1,3]dioxol-4-yl}methyl 2-methylpropanoate (hydroxylamino molnupiravir acetonide) (synthesis related impurity),
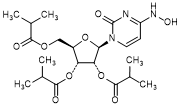
H. (2R,3R,4R,5R)-2-[4-(Hydroxyamino)-2-oxopyrimidin-1(2H)-yl]-5-{[(2-methylpropanoyl)oxy]methyl}oxolane-3,4-diyl bis(2-methylpropanoate) (molnupiravir triester, triacylated molnupiravir) (synthesis related impurity),
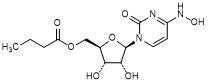
I. {(2R,3S,4R,5R)-3,4-Dihydroxy-5-[4-(hydroxyamino)-2-oxopyrimidin-1(2H)-yl]oxolan-2-yl}methyl butanoate (molnupiravir n-butanoyl analog) (synthesis related impurity),
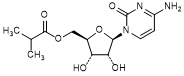
L. Cytidine 5′-O-(2-methylpropanoate) or [(2R,3S,4R,5R)-5-(4-Amino-2-oxopyrimidin-1(2H)-yl)-3,4-dihydroxyoxolan-2-yl]methyl 2-methylpropanoate (cytidine isobutyl ester) (synthesis related impurity).