Monographs: Pharmaceutical substances: Morphine sulfate (Morphini sulfas)
Molecular formula. (C17H19NO3)2,H2SO4,5H2O
Relative molecular mass. 758.8
Graphic formula.
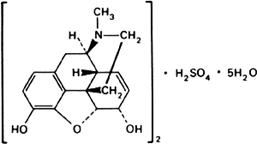
Chemical name. 7,8-Didehydro-4,5α-epoxy-17-methylmorphinan-3,6α-diol sulfate (2:1) (salt) pentahydrate; CAS Reg. No. 6211-15-0.
Description. White, feathery needles of a white, crystalline powder or cubical masses; odourless.
Solubility. Soluble in 20 parts of water; slightly soluble in ethanol (~750 g/l) TS; practically insoluble in ether R.
Category. Analgesic.
Storage. Morphine sulfate should be kept in a tightly closed container, protected from light.
Additional information. Morphine sulfate loses water of hydration on exposure to air and darkens in colour on prolonged exposure to light.
Requirements
Definition. Morphine sulfate contains not less than 98.0% and not more than 101.0% of (C17H19NO3)2,H2SO4, calculated with reference to the dried substance.
Identity tests
A. To 5 mL of a 1 mg/mL solution add 3 drops of a freshly prepared potassium ferricyanide (10 g/l) TS and 1 drop of ferric chloride (25 g/l) TS; a bluish green colour is produced.
B. To 5 mL of a 1 mg/mL solution add 1 mL of hydrogen peroxide (~60 g/l) TS, 1 mL of ammonia (~100 g/l) TS and 1 drop of copper (II) sulfate (80 g/l) TS; a transient red colour is produced.
C. To about 1 mg add 0.5 mL of sulfuric acid (~1760 g/l) TS containing 1 drop of formaldehyde TS; a purple colour is produced, which changes quickly to violet.
D. A 20 mg/mL solution yields reaction A described under 2.1 General identification tests as characteristic of sulfates.
Specific optical rotation. Use a 20 mg/mL solution and calculate with reference to the dried substance;  = -106° to -110°.
= -106° to -110°.
Sulfated ash. Not more than 1.0 mg/g.
Loss on drying. Dry at 145°C for 1 hour; it loses not less than 90 mg/g and not more than 120 mg/g.
Acidity. Dissolve 0.2 g in 10 mL of carbon-dioxide-free water R, and titrate with sodium hydroxide (0.02 mol/l) VS, using methyl red/ethanol TS as indicator; not more than 0.2 mL is required to obtain the midpoint of the indicator (orange).
Meconate. Dissolve 0.2 g in 5 mL of water, add 5 mL of hydrochloric acid (~70 g/l) TS and a few drops of ferric chloride (25 g/l) TS; no red colour is produced.
Related alkaloids. Transfer about 0.5 g, accurately weighed, to a separator, add 15 mL of water, 2 mL of sodium hydroxide (10 g/l) TS, and 10 mL of chloroform R. Shake, allow to separate, and transfer the chloroform layer to another separator. Repeat the extraction with further quantities of chloroform R, each of 10 mL. Wash the combined chloroform solutions with 4 mL of sodium hydroxide (10 g/l) TS and twice with water, using 5 mL each time. Separate the chloroform layer and evaporate it carefully to dryness on a water-bath. Add to the residue thus obtained 10 mL of sulfuric acid (0.01 mol/l) VS, heat until dissolved, cool, add 1 drop of methyl red/ethanol TS, and titrate the excess acid with sodium hydroxide (0.02 mol/l) VS; not less than 8.75 mL is required to obtain the midpoint of the indicator (orange).
Noscapine. Dissolve 0.05 g in 2 mL of sulfuric acid (~1760 g/l) TS and heat the solution on a water-bath; no violet colour is produced.
Assay. Dissolve about 0.6 g, accurately weighed, in 30 mL of glacial acetic acid R1, and titrate with perchloric acid (0.1 mol/l) VS, determining the end-point potentiometrically as described under 2.6 Non-aqueous titration. Method A. Each mL of perchloric acid (0.1 mol/l) VS is equivalent to 66.88 mg of (C17H19NO3)2,H2SO4.
Additional requirements for Morphine sulfate for parenteral use
Complies with the monograph for "Parenteral preparations".
Bacterial endotoxins. Carry out the test as described under 3.4 Test for bacterial endotoxins; contains not more than 14.29 IU of endotoxin RS per mg.