Monographs: Pharmaceutical substances: Neostigmine bromide (Neostigmini bromidum)
Molecular formula. C12H19BrN2O2
Relative molecular mass. 303.2
Graphic formula.
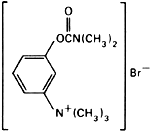
Chemical name. (m-Hydroxyphenyl)trimethylammonium bromide dimethylcarbamate; 3-[[(dimethylamino)carbonyl]oxy]-N,N,N-trimethylbenzenaminium bromide; CAS Reg. No. 114-80-7.
Description. Colourless crystals or a white, crystalline powder; odourless.
Solubility. Very soluble in water; freely soluble in ethanol (~750 g/l) TS; practically insoluble in ether R.
Category. Cholinergic.
Storage. Neostigmine bromide should be kept in a tightly closed container, protected from light.
Requirements
Definition. Neostigmine bromide contains not less than 98.0% and not more than 101.0% of C12H19BrN2O2, calculated with reference to the dried substance.
Identity tests
A. Heat 0.05 g with 0.4 g of potassium hydroxide R and 2 mL of ethanol (~750 g/l) TS on a water-bath for 3 minutes. Replace the evaporated ethanol, cool, and add 2 mL of water and 2 mL of diazobenzenedisulfonic acid TS; a red colour is produced.
B. To a solution of 0.1 g in 5 mL of water add 15 mL of trinitrophenol (7 g/l) TS, wash the precipitate with water, and dry at 105°C; melting temperature, about 185°C (picrate).
C. A 20 mg/mL solution yields reaction A described under 2.1 General identification tests as characteristic of bromides.
Sulfates. Dissolve 2.5 g in 40 mL of water and proceed as described under 2.2.2 Limit test for sulfates; the sulfate content is not more than 0.2 mg/g.
Sulfated ash. Not more than 1.5 mg/g.
Loss on drying. Dry to constant weight at 105°C; it loses not more than 20 mg/g.
Acidity. Dissolve 0.2 g in 20 mL of carbon-dioxide-free water R and titrate to pH 7.0 with sodium hydroxide (0.02 mol/l) VS; not more than 0.1 mL is required.
3-Hydroxyphenyltrimethylammonium bromide. The absorbance of a 1-cm layer of a freshly prepared 5.0 mg/mL solution in sodium carbonate (10 g/l) TS at 294 nm is not more than 0.2 (preferably use 2-cm cells for the measurement and calculate the absorbance of a 1-cm layer).
Assay.
In order to avoid overheating in the reaction medium, mix thoroughly throughout the titration and stop the titration immediately after the end-point has been reached.
Dissolve 0.230 g in 2 mL of anhydrous formic acid R and add 50 mL of acetic anhydride R. Carry out a potentiometric titration using perchloric acid (0.1 mol/L) VS, as described under 2.6 Non-aqueous titration.
1 mL of 0.1 M perchloric acid (0.1 mol/L) is equivalent to 30.32 mg of C12H19BrN2O2.