Monographs: Pharmaceutical substances: Niclosamide (Niclosamidum)
Niclosamide, anhydrous
Niclosamide monohydrate
Molecular formula. C13H8Cl2N2O4 (anhydrous); C13H8Cl2N2O4.H2O (monohydrate)
Relative molecular mass. 327.1 (anhydrous); 345.1 (monohydrate)
Graphic formula.
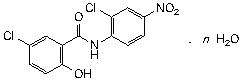
n: 0 Niclosamide, anhydrous; 1 Niclosamide monohydrate
Chemical name. 5-chloro-N-(2-chloro-4-nitrophenyl)-2-hydroxybenzamide; 2',5-dichloro-4'-nitrosalicylanilide; CAS Reg. No. 50-65-7 (anhydrous).
5-chloro-N-(2-chloro-4-nitrophenyl)-2-hydroxybenzamide monohydrate; 2',5-dichloro-4'-nitrosalicylanilide hydrate (1:1); CAS Reg. No. 73360-56-2 (monohydrate).
Description. A cream-coloured, crystalline powder; odourless.
Solubility. Practically insoluble in water; soluble in 150 parts of ethanol (~750 g/L) TS; slightly soluble in ether R and acetone R.
Category. Taeniacide.
Storage. Niclosamide should be kept in a tightly closed container.
Labelling. The designation on the container of Niclosamide should state whether the substance is the monohydrate or is in the anhydrous form.
Additional information. Anhydrous Niclosamide is hygroscopic. Niclosamide monohydrate may exhibit polymorphism.
Requirements
Definition. Niclosamide contains not less than 98.0% and not more than 100.5% of C13H8Cl2N2O4, calculated with reference to the dried substance.
Identity tests
- Either test A alone or tests B and C may be applied.
A. Carry out the examination as described under 1.7 Spectrophotometry in the infrared region. For the anhydrous substance the infrared absorption spectrum is concordant with the spectrum obtained from niclosamide RS, which has been dried at 100–105 °C for 4 h, or with the reference spectrum of niclosamide. For the monohydrate dry the substance to be examined and niclosamide RS at 100–105 °C for 4 h. The infrared absorption spectrum of the dried substance is concordant with the spectrum obtained from the dried niclosamide RS or with the reference spectrum of niclosamide.
B. Dissolve 1 mg in 2 mL of dimethylformamide R and add 2 drops of potassium hydroxide/ethanol TS1; a strong red colour is produced.
C. Dissolve 0.1 g in 1 mL of acetic anhydride R and boil for 10 minutes. Cool and add 10 mL of water. Collect the precipitate on a filter, wash with water, recrystallize from ethanol (~750 g/L) TS and dry at 105 °C; melting temperature about 178 °C (acetyl-derivative).
Sulfated ash. Not more than 1.0 mg/g.
Loss on drying
Dry to constant weight at 105 °C. Anhydrous Niclosamide loses not more than 5.0 mg/g. Niclosamide monohydrate loses not less than 40 mg/g and not more than 60 mg/g.
Acidity or alkalinity. Boil 0.8 g in 40 mL of water for 1 minute and filter. To 10 mL of the filtrate add 2 drops of phenolphthalein/ethanol TS and 0.2 mL of carbonate-free sodium hydroxide (0.01 mol/L) VS; a red colour is produced. Add 5 drops of methyl red/ethanol TS and 0.4 mL of hydrochloric acid (0.01 mol/L) VS; the colour of the solution changes from red to orange.
2-Chloro-4-nitroaniline. Boil 0.1 g with 20 mL of methanol R for 2 minutes, cool, add sufficient hydrochloric acid (1 mol/L) VS to produce 50 mL and filter. To 10 mL of the filtrate add 1.0 mL of sodium nitrite (3 g/L) TS and allow to stand for 10 minutes; add 1 mL of ammonium sulfamate (25 g/L) TS, shake, allow to stand for 10 minutes and add 1 mL of N-(1-naphthyl)ethylenediamine hydrochloride (5 g/L) TS. Treat similarly 10 μg of 2-chloro-4-nitroaniline R. The colour produced in the test solution is not more intense than that of the reference solution when compared as described under 1.11.1 Colour of liquids.
5-Chlorosalicylic acid. Boil 0.5 g with 10 mL of water for 2 minutes, cool, filter and add to the filtrate a few drops of ferric chloride (25 g/L) TS; no red or violet colour is produced.
Assay
Dissolve about 0.3 g, accurately weighed, in 60 mL of dimethylformamide R and titrate with tetrabutylammonium hydroxide (0.1 mol/L) VS determining the end-point potentiometrically as described under 2.6 Non-aqueous titration, Method B. Each mL of tetrabutylammonium hydroxide (0.1 mol/L) VS is equivalent to 32.71 mg of C13H8Cl2N2O4.