Monographs: Pharmaceutical substances: Nicotinamide (Nicotinamidum)
Molecular formula. C6H6N2O
Relative molecular mass. 122.1
Graphic formula.
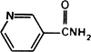
Chemical name. 3-Pyridinecarboxamide; 3-pyridinecarboxylic acid amide; CAS Reg. No. 98-92-0.
Description. Colourless crystals or a white, crystalline powder; odourless or almost odourless.
Solubility. Soluble in 1 part of water and 2 parts of ethanol (~750 g/l) TS; slightly soluble in ether R.
Category. Vitamin.
Storage. Nicotinamide should be kept in a well-closed container.
Requirements
Definition. Nicotinamide contains not less than 99.0% and not more than 101.0% of C6H6N2O, calculated with reference to the dried substance.
Identity tests
• Either test A alone or tests B and C may be applied.
A. Carry out the examination as described under 1.7 Spectrophotometry in the infrared region. The infrared absorption spectrum is concordant with the spectrum obtained from nicotinamide RS or with the reference spectrum of nicotinamide.
B. Dissolve 10 mg in 10 mL of water. To 2 mL add 2 mL of thiocyanate reagent, obtained by adding, drop by drop, ammonium thiocyanate (0.1 mol/l) VS to bromine TS1 until the yellow coloration disappears. Then add 3 mL of aniline (25 g/l) TS and shake; a yellow colour is produced.
C. Boil gently 0.1 g with 1 mL of sodium hydroxide (~80 g/l) TS in a test-tube; ammonia, perceptible by its odour, is evolved.
Melting range. 128-131°C.
Heavy metals. Use 1.0 g for the preparation of the test solution as described under 2.2.3 Limit test for heavy metals, Procedure 1; determine the heavy metals content according to Method A; not more than 30 μg/g.
Clarity and colour of solution. A solution of 2.5 g in 10 mL of water is clear and not more intensely coloured than standard colour solution Yw2 when compared as described under 1.11.1 Colour of liquids.
Sulfated ash. Not more than 1.0 mg/g.
Loss on drying. Dry to constant weight at ambient temperature under reduced pressure (not exceeding 0.6 kPa or about 5 mm of mercury) over silica gel, desiccant, R or phosphorus pentoxide R; it loses not more than 5.0 mg/g.
pH value. pH of a 0.05 g/mL solution in carbon-dioxide-free water R, 6.0-8.0.
Related substances. Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography, using silica gel R2 as the coating substance and a mixture of 48 volumes of chloroform R, 10 volumes of water and 45 volumes of dehydrated ethanol R as the mobile phase. Apply separately to the plate 5 μl of each of 2 solutions in a mixture of equal volumes of ethanol (~750 g/l) TS and water containing (A) 0.12 g of the test substance per mL and (B) 0.30 mg of the test substance per mL. After removing the plate from the chromatographic chamber, allow it to dry in air, and examine the chromatogram in ultraviolet light (254 nm). Any spot obtained with solution A, other than the principal spot, is not more intense than that obtained with solution B.
Assay. Dissolve about 0.25 g, accurately weighed, in 20 mL of glacial acetic acid R1, add 5 mL of acetic anhydride R, and titrate with perchloric acid (0.1 mol/l) VS as described under 2.6 Non-aqueous titration. Method A. Each mL of perchloric acid (0.1 mol/l) VS is equivalent to 12.21 mg of C6H6N2O.