Monographs: Pharmaceutical substances: Propylene glycol (Propyleneglycolum)
Graphic formula.
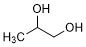
Molecular formula. C3H8O2
Relative molecular mass. 76.09
Chemical name. Propane-1,2-diol (IUPAC); 1,2-Propanediol (CAS); Propylene glycol.
CAS Registry Number. 57-55-6.
Description. A colourless, clear, and viscous liquid; odourless.
Miscibility. Miscible with water and ethanol (~750 g/l) TS.
Category. Solvent; humectant.
Storage. Propylene glycol should be kept in a tightly closed container.
Additional information. Propylene glycol is hygroscopic. Boiling range, 185-189 °C. The substance is at risk for ethylene glycol (EG) or diethylene glycol (DEG) contamination and must therefore be tested for these contaminants before it is used in the production of medicines. The International Pharmacopoeia provides analytical test procedures for the detection of EG and DEG in liquid preparations for oral use in the Supplementary Information section, which are transferable to pharmaceutical substances. Pharmaceutical manufacturers are expected to use the gas chromatography method to test for DEG/EG contamination.
Requirements
Identity test
Dissolve 0.1 mL in sufficient water to produce 100 mL, dilute 1 mL to 10 mL, and place 0.5 mL of this solution in a test-tube. Cool in ice, add 5 mL of a cooled mixture of 10 mL of water and 90 mL of sulfuric acid (~1760 g/l) TS, heat on a water-bath at 70 °C for 10 minutes, and cool again. Add 0.2 mL of triketohydrindene/sodium metabisulfite TS; a violet colour slowly appears.
Refractive index. 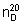 = 1.431 - 1.433
= 1.431 - 1.433
Relative density. 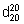 = 1.035 - 1.040
= 1.035 - 1.040
Heavy metals. Use 4 mL for the preparation of the test solution as described under 2.2.3 Limit test for heavy metals, Procedure 1; determine the heavy metals content according to Method A; not more than 5 μg/g.
Clarity and colour of solution. Propylene glycol should be clear and colourless.
Sulfated ash. Use 50 g; the residue weighs not more than 5 mg (0.1 mg/g).
Water. Determine as described under 2.8 Determination of water by the Karl Fischer method, Method A, using 5 g; the water content is not more than 2.0 mg/g.
Acidity. To 10 mL add 40 mL of water and 0.1 mL of bromothymol blue/ethanol TS; the solution is greenish yellow. Titrate with sodium hydroxide (0.1 mol/l) VS; not more than 0.05 mL is required to obtain the midpoint of the indicator (blue).
Oxidizing substances. To 10 mL add 5 mL of water, 2 mL of potassium iodide (80 g/l) TS, and 2 mL of sulfuric acid (~100 g/l) TS, and allow to stand in a stoppered flask protected from light for 15 minutes. Titrate with sodium thiosulfate (0.05 mol/l) VS, using starch TS as indicator; not more than 0.2 mL is required.
Reducing substances. To 1 mL add 1 mL of ammonia (~100 g/l) TS and heat in a water-bath at 60 °C for 5 minutes; the solution is yellow. Without delay add 0.15 mL of silver nitrate (0.1 mol/l) VS and allow to stand for 5 minutes; the colour and aspect of the solution remain unchanged.