Monographs: Pharmaceutical substances: Quinine hydrochloride (Quinini hydrochloridum)
Molecular formula. C20H24N2O2,HCl,2H2O
Relative molecular mass. 396.9
Graphic formula.
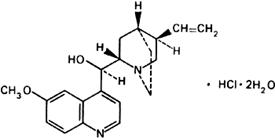
Chemical name. (8α,9R)-6'-Methoxycinchonan-9-ol monohydrochloride (salt) dihydrate; (8α,9R)-9-hydroxy-6'-methoxycinchonan hydrochloride (1:1) (salt) dihydrate; CAS Reg. No. 6119-47-7.
Description. Silky, colourless crystals, often grouped in clusters; odourless.
Solubility. Soluble in water; freely soluble in ethanol (~750 g/l) TS; very slightly soluble in ether R.
Category. Antimalarial.
Storage. Quinine hydrochloride should be kept in a well-closed container, protected from light.
Additional information. Quinine hydrochloride has a very bitter taste.
Requirements
Definition. Quinine hydrochloride contains not less than 98.5% and not more than 101.0% of total alkaloids, calculated as C20H24N2O2,HCl and with reference to the dried substance.
Identity tests
A. Dissolve 0.1 g in 2.5 mL of water; the solution is not fluorescent. Dilute 0.5 mL of this solution to 100 mL with water and add 2 drops of sulfuric acid (~100 g/l) TS; a strong blue fluorescence is produced.
B. To 5 mL of a 1 mg/mL solution add 2-3 drops of bromine TS1 and 5 drops of ammonia (~100 g/l) TS; an emerald-green colour is produced.
C. A 10 mg/mL solution yields reaction B described under 2.1 General identification tests as characteristic of chlorides.
Specific optical rotation. Use a 20 mg/mL solution in hydrochloric acid (0.1 mol/l) VS and calculate with reference to the dried substance;  = -240° to -258°.
= -240° to -258°.
Barium. To 15 mL of a 0.3 g/mL solution add 1 mL of sulfuric acid (~100 g/l) TS; the solution remains clear for not less than 15 minutes.
Sulfates. Dissolve 0.5 g in 20 mL of water and proceed as described under 2.2.2 Limit test for sulfates; the sulfate content is not more than 1 mg/g.
Clarity and colour of solution. A solution of 0.10 g in 10 mL of water is clear and colourless.
Sulfated ash. Not more than 1.0 mg/g.
Loss on drying. Dry to constant weight at 105°C; it loses not less than 60 mg/g and not more than 100 mg/g.
pH value. pH of a 10 mg/mL solution in carbon-dioxide-free water R, 6.0-7.0.
Related cinchona alkaloids. Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography, using silica gel R1 as the coating substance and a mixture of 20 volumes of toluene R, 12 volumes of ether R, and 5 volumes of diethylamine R as the mobile phase. Apply separately to the plate 4 μl of each of 2 solutions in methanol R containing (A) 10 mg of the test substance per mL and (B) 0.25 mg of cinchonidine R per mL. After removing the plate from the chromatographic chamber, heat it at 105°C for 30 minutes, allow it to cool, spray with potassium iodoplatinate TS, and examine the plate in daylight. Any spot obtained with solution A, other than the principal spot, is not more intense than that obtained with solution B.
Dihydroquinine. Dissolve about 0.2 g, accurately weighed, in 20 mL of water. Add 0.5 g of potassium bromide R, 15 mL of hydrochloric acid (~70 g/l) TS and 0.1 mL of methyl red/ethanol TS. Titrate with potassium bromate (0.0167 mol/l) VS until a yellow colour is produced. Add 0.5 g of potassium iodide R in 200 mL of water, stopper the flask, and allow to stand in the dark for 5 minutes. Titrate the iodine liberated by excess potassium bromate in the solution with sodium thiosulfate (0.1 mol/l) VS, adding 2 mL of starch TS when the solution has reached a light yellow coloration. Each mL of potassium bromate (0.0167 mol/l) VS is equivalent to 18.04 mg of C20H24N2O2,HCl. Express the results of both the above determination and the assay in percentages, calculated with reference to the dried substance. The difference between the two is not more than 10%.
Assay. Dissolve 0.250 g in 50 mL of dehydrated ethanol R and add 5.0 mL of hydrochloric acid (0.01 mol/L) VS. Carry out a potentiometric titration using sodium hydroxide (0.1 mol/L) VS. Read the volume added between the two inflexion points.
1 mL of 0.1 M sodium hydroxide is equivalent to 36.09 mg of C20H24N2O2,HCl.