Monographs: Pharmaceutical substances: Salicylic acid (Acidum salicylicum)
Molecular formula. C7H6O3
Relative molecular mass. 138.1
Graphic formula.
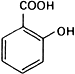
Chemical name. 2-Hydroxybenzoic acid; CAS Reg. No. 69-72-7.
Description. Colourless crystals, usually needle-like, or a white, crystalline powder; odourless.
Solubility. Slightly soluble in water; soluble in 4 parts of ethanol (~750 g/l) TS and in 3 parts of ether R.
Category. Keratolytic.
Storage. Salicylic acid should be kept in a well-closed container.
Requirements
Definition. Salicylic acid contains not less than 99.0% and not more than 101.0% of C7H6O3, calculated with reference to the dried substance.
Identity test
Dissolve 0.14 g in 1 mL of sodium hydroxide (1 mol/l) VS and add 5 mL of water; this solution yields the reaction described under 2.1 General identification tests as characteristic of salicylates.
Melting range. 158-161°C.
Heavy metals. Use 2.0 g and 15 mL of ethanol (~750 g/l) TS for the preparation of the test solution as described under 2.2.3 Limit test for heavy metals. Procedure 2; determine the heavy metals content according to Method A; not more than 20 μg/g.
Chlorides. Dissolve 1.7 g in 40 mL of boiling water, cool and filter. Add 2 mL of nitric acid (~130 g/l) TS to the filtrate and proceed as described under 2.2.1 Limit test for chlorides; the chloride content is not more than 0.15 mg/g.
Sulfates. Dissolve 2.5 g in 40 mL of boiling water, cool, filter, and proceed with the filtrate as described under 2.2.2 Limit test for sulfates; the sulfate content is not more than 0.2 mg/g.
Solution in ethanol. A solution of 1.0 g in 10 mL of ethanol (~750 g/l) TS is clear and colourless.
Sulfated ash. Not more than 1.0 mg/g.
Loss on drying. Dry to constant weight over silica gel, desiccant, R at ambient temperature; it loses not more than 5.0 mg/g.
Assay. Dissolve about 0.3 g, accurately weighed, in 15 mL of neutralized ethanol TS and add 20 mL of water. Titrate with carbonate-free sodium hydroxide (0.1 mol/l) VS, using phenolphthalein/ethanol TS as indicator. Repeat the operation without the substance being examined and make any necessary corrections. Each mL of carbonate-free sodium hydroxide (0.1 mol/l) VS is equivalent to 13.81 mg of C7H6O3.