Monographs: Pharmaceutical substances: Testosterone propionate (Testosteroni propionas)
Molecular formula. C22H32O3
Relative molecular mass. 344.5
Graphic formula.
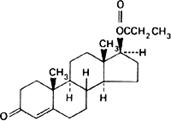
Chemical name. 17β-(1-Oxopropoxy)androst-4-en-3-one; 17β-hydroxyandrost-4-en-3-one propionate; CAS Reg. No. 57-85-2.
Description. Colourless or slightly yellowish crystals or a white or slightly yellowish powder; odourless.
Solubility. Practically insoluble in water; freely soluble in ethanol (~750 g/l) TS and ether R; soluble in vegetable oils.
Category. Androgen.
Storage. Testosterone propionate should be kept in a well-closed container, protected from light.
Requirements
Definition. Testosterone propionate contains not less than 97.0% and not more than 102.0% of C22H32O3, calculated with reference to the dried substance.
Identity tests
• Either test A or tests B and C may be applied.
A. Carry out the examination as described under 1.7 Spectrophotometry in the infrared region. The infrared absorption spectrum is concordant with the spectrum obtained from testosterone propionate RS or with the reference spectrum of testosterone propionate.
B. See the test described below under "Related substances". The principal spot obtained with solution C corresponds in position, appearance, and intensity with that obtained with solution D.
C. Melting temperature, about 121°C.
Specific optical rotation. Use a 10 mg/mL solution in dioxan R;  = +81° to +91°.
= +81° to +91°.
Solution in ethanol. A solution of 0.50 gin 10 mL of ethanol (~750 g/L) TS is clear and not more intensely coloured than standard colour solution Yw2 when compared as described under 1.11.1 Colour of liquids.
Loss on drying. Dry to constant weight at 105°C; it loses not more than 5.0 mg/g.
Related substances. Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography, using silica gel R1 as the coating substance and a mixture of 92 volumes of dichloroethane R, 8 volumes of methanol R, and 0.5 volumes of water as the mobile phase. Apply separately to the plate 5 μl of each of 2 solutions in a mixture of 9 volumes of chloroform R and 1 volume of methanol R containing (A) 20 mg of the test substance per mL; (B) 0.20 mg of the test substance per mL; (C) 1.0 mg of the test substance per mL and (D) 1.0 mg of testosterone propionate RS per mL. After removing the plate from the chromatographic chamber, allow it to dry in air and heat at 110°C for 10 minutes. Spray the hot plate with sulfuric acid/ethanol TS, again heat it at 110°C for 10 minutes, and examine the chromatogram in ultraviolet light (365 nm). Any spot obtained with solution A, other than the principal spot, is not more intense than that obtained with solution B.
Assay. Dissolve about 20 mg, accurately weighed, in sufficient ethanol (~750 g/l) TS to produce 100 mL; dilute 5.0 mL of this solution to 100 mL with the same solvent. Measure the absorbance of a 1-cm layer of the diluted solution at the maximum at about 241 nm. Calculate the amount of C22H32O3 in the substance being tested by comparison with testosterone propionate RS, similarly and concurrently examined. In an adequately calibrated spectrophotometer the absorbance of the reference solution should be 0.50 ± 0.03.