Monographs: Pharmaceutical substances: Tetracycline hydrochloride (Tetracyclini hydrochloridum)
Molecular formula. C22H24N2O8, HCl
Relative molecular mass. 480.9
Graphic formula.
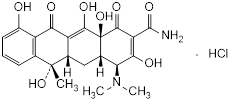
Chemical name. (4S,4aS,5aS,6S,12aS)-4-(Dimethylamino)-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydronaphtacene-2-carboxamide monohydrochloride (IUPAC); 2-Naphthacenecarboxamide, 4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-, hydrochloride (1:1), (4S,4aS,5aS,6S,12aS)- (CAS). CAS Reg. No. 64-75-5.
Description. A yellow, crystalline powder.
Solubility. Soluble in 10 parts of water R, slightly soluble in ethanol (~750 g/l) TS; practically insoluble in acetone R. Dissolves in solutions of alkali hydroxides and carbonates. Solutions in water R become turbid on standing, owing to the precipitation of tetracycline.
Category. Antibiotic.
Storage. Tetracycline hydrochloride should be kept in a tightly closed container, protected from light.
Additional information. Tetracycline hydrochloride decomposes rapidly in solutions below pH 2, and less rapidly in solutions above pH 7. Even in the absence of light, Tetracycline hydrochloride is gradually degraded on exposure to a humid atmosphere, the decomposition being faster at higher temperatures. Tetracycline hydrochloride is produced by certain strains of Streptomyces aerofaciens or obtained by any other means.
Requirements
Definition. Tetracycline hydrochloride contains not less than 95.0% and not more than 102.0% of C22H24N2O8,HCl, calculated with reference to the dried substance.
Identity tests
- Either tests A, E or tests B, D and E or tests C, D and E may be applied.
A. Carry out the examination as described under 1.7 Spectrophotometry in the infrared region. The infrared absorption spectrum is concordant with the spectrum obtained from tetracycline hydrochloride RS or with the reference spectrum of tetracycline hydrochloride RS.
B. Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography using silica gel R7 as the coating substance and a mixture of acetonitrile R, methanol R and a 63 g/L solution of oxalic acid R previously adjusted to pH 2 with ammonia (~260 g/L) TS (20:20:60 V/V/V) as the mobile phase. Apply separately to the plate 1 µL of each of the following three solutions in methanol R containing (A) 0.5 mg of the test substance per mL, (B) 0.5 mg of tetracycline hydrochloride RS per mL and (C) 0.5 mg of tetracycline hydrochloride RS, 0.5 mg of demeclocycline hydrochloride R and 0.5 mg oxytetracycline hydrochloride R per mL. Develop the plate for a distance of 15 cm. After removing the plate from the chromatographic chamber, allow it to dry in air or in a current of air. Examine the chromatogram under ultraviolet light (254 nm). The test is not valid unless the chromatogram obtained with solution (C) shows three clearly separated spots. The principal spot in the chromatogram obtained with solution (A) corresponds in position, appearance and intensity with the spot due to tetracycline in the chromatogram obtained with solution (B).
C. Carry out the test as described under 1.14.4 High-performance liquid chromatography using the conditions given under “Assay”. The retention time of the principal peak in the chromatogram obtained with solution (1) corresponds to the retention time of the tetracycline peak in the chromatogram obtained with solution (2).
D. To about 1 mg of the test substance, add 2 mL of sulfuric acid (~1760 g/l) TS; a red-violet colour is produced which on the addition of 0.1 mL of water R changes to yellow.
E. A 0.05 g/mL solution yields reaction B described under 2.1 General identification tests as characteristic of chlorides.
Specific optical rotation (1.4). Use a 10 mg/mL solution in hydrochloric acid (0.01 mol/L) VS;  = -240 to -255 with reference to the dried substance.
= -240 to -255 with reference to the dried substance.
Loss on drying. Dry at 60 °C under reduced pressure (not exceeding 0.6 kPa or about 5 mm of mercury) over phosphorus pentoxide R for 3 hours; it loses not more than 20 mg/g.
pH value (1.13). pH of a 10 mg/mL solution, 1.8-2.8.
Sulfated ash (2.3) . Not more than 5.0 mg/g.
Related substances. Carry out the test as described under 1.14.1 Chromatography, High-performance liquid chromatography, using the conditions given below under “Assay”.
Use solution (1) as described under “Assay”. Prepare the following additional solutions using mobile phase A as diluent. For solution (3), dissolve 12.5 mg of anhydrotetracycline hydrochloride RS and dilute to 50.0 mL. For solution (4), dissolve 12.5 mg of 4-epi-tetracycline hydrochloride RS and dilute to 50.0 ml. For solution (5), dissolve 12.5 mg of 4-epi-anhydrotetracycline hydrochloride RS and dilute to 50.0 ml. For solution (6), transfer 10.0 mL of solution (1) and 5.0 mL each of solutions (3), (4) and (5) to a 50 mL volumetric flask, mix and dilute to volume. For solution (7), dilute 1 volume of solution (3) to 500 volumes. For solution (8), dilute 1 volume of solution (4) to 100 volumes. For solution (9), dilute 1 volume of solution (5) to 500 volumes.
Inject alternately 10 μL each of solutions (1), (6), (7), (8) and (9).
The following peaks are eluted at the following relative retention with reference to tetracycline (retention time about 5 minutes): impurity A (4-epi-tetracycline) about 0.9; impurity B (2-acetyl-2-decarbamoyltetracycline) about 1.1; impurity D (4-epi-anhydrotetracycline) about 1.5; and impurity C (anhydrotetracycline) about 1.7.
The assay is not valid unless, in the chromatogram obtained with solution (6), the resolution between 4-epi-tetracycline and tetracycline is at least 2.5 and the resolution between 4-epi-anhydrotetracycline and anhydrotetracycline is at least 2.5.
In the chromatogram obtained with solution (1):
- the area of any peak corresponding to impurity A is not greater than 1.5 times the area of the peak due to impurity A (4-epi-tetracycline) in the chromatogram obtained with solution (8) (3.0%);
- the area of any peak corresponding to impurity B (2-acetyl-2-decarbamolyltetracycline) is not greater than 0.75 times the area of the peak due to impurity A (4-epi-tetracycline) in the chromatogram obtained with solution (8) (1.5%);
- the area of any peak corresponding to impurity C is not greater than 1.25 times the area of the peak due to impurity C (anhydrotetracycline) in the chromatogram obtained with solution (7) (0.5%); and
- the area of any peak corresponding to impurity D is not greater than 1.25 times the area of the peak due to impurity D (4-epi-anhydrotetracycline) in the chromatogram obtained with solution (9) (0.5%).
Assay. Carry out the test as described under 1.14.1 Chromatography, High-performance liquid chromatography using a stainless steel column (15 cm x 4.6 mm) packed with particles of base-deactivated silica gel, the surface of which has been modified with chemically-bonded octadecylsilyl groups (3 µm).
Use the following conditions for gradient elution:
- Mobile phase A: 1 volume of phosphoric acid (~1440g/L) TS in 1000 volumes of water R;
- Mobile phase B: acetonitrile R.
|
Time (min) |
Mobile phase A (% v/v) |
Mobile phase B (% v/v) |
Comment |
|
0-7.5 |
85 to 60 |
15 to 40 |
Linear gradient |
|
7.5-7.6 |
60 to 85 |
40 to 15 |
Return to initial conditions |
|
7.6-10 |
85 |
15 |
re-equilibration |
Operate with a flow rate of 1.0 mL per minute. As a detector, use an ultraviolet spectrophotometer set at a wavelength of 280 nm. Maintain the column temperature at 50 °C and the autosampler temperature at 10 °C.
Prepare the following solutions using mobile phase A as diluent. For solution (1), dissolve 25.0 mg of the test substance and dilute to 200.0 mL. For solution (2), dissolve 25.0 mg of tetracycline hydrochloride RS and dilute to 200.0 mL.
Inject alternately 10 μL each of solutions (1) and (2).
Measure the areas of the peak responses obtained in the chromatograms from solutions (1) and (2) and calculate the percentage content of C22H24N2O8, HCl using the declared content of C22H24N2O8, HCl in tetracycline hydrochloride RS.
Bacterial endotoxins. If intended for use in the manufacture of a parenteral dosage form without a further appropriate procedure for the removal of bacterial endotoxins, carry out the test as described under 3.4 Test for bacterial endotoxins; contains not more than 0.5 IU of endotoxin RS per mg of tetracycline hydrochloride.
Impurities.
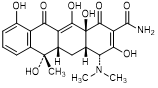
A. (4R,4aS,5aS,6S,12aS)-4-(dimethylamino)-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydronaphtacene-2-carboxamide (4-epi-tetracycline).
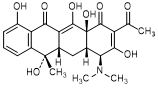
B. (4S,4aS,5aS,6S,12aS)-2-acetyl-4-(dimethylamino)-3,6,10,12,12a-pentahydroxy-6-methyl-4a,5a,6,12a-tetrahydronaphtacene-1,11(4H,5H)-dione (2-acetyl-2-decarbamoyltetracycline).
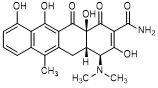
C. (4S,4aS,12aS)-4-(dimethylamino)-3,10,11,12a-tetrahydroxy-6-methyl-1,12-dioxo-1,4,4a,5,12,12a-hexahydronaphtacene-2-carboxamide (anhydrotetracycline).
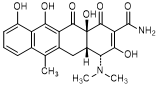
D. (4R,4aS,12aS)-4-(dimethylamino)-3,10,11,12a-tetrahydroxy-6-methyl-1,12-dioxo-1,4,4a,5,12,12a-hexahydronaphtacene-2-carboxamide (4-epi-anhydrotetracycline).
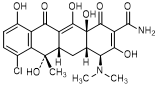
E. (4S,4aS,5aS,6S,12aS)-7-chloro-4-(dimethylamino)-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydronaphtacene-2-carboxamide (chlortetracycline).