Monographs: Pharmaceutical substances: Thiamine hydrochloride (Thiamini hydrochloridum)
Molecular formula. C12H17ClN4OS,HCl
Relative molecular mass. 337.3
Graphic formula.
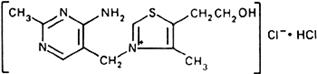
Chemical name. Thiamine chloride, hydrochloride; 3-[(4-amino-2-methyl-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methylthiazolium chloride, mono-hydrochloride; CAS Reg. No. 67-03-8.
Description. Colourless crystals or a white or yellowish white, crystalline powder; odour, slight and characteristic.
Solubility. Soluble in 1 part of water and in 100 parts of ethanol (~750 g/l) TS; practically insoluble in acetone R and ether R.
Category. Component of vitamin B.
Storage. Thiamine hydrochloride should be kept in a tightly closed, non-metallic container, protected from light.
Additional information. Even in the absence of light, Thiamine hydrochloride is gradually degraded on exposure to a humid atmosphere, the decomposition being faster at higher temperatures. When exposed to air, the anhydrous product rapidly absorbs about 4 g of water per 100 g. Melting temperature, about 248 °C with some decomposition. In solution at pH 4.0 or less, it loses its activity only very slowly. Neutral and alkaline solutions deteriorate rapidly, especially in contact with air.
Requirements
Definition. Thiamine hydrochloride contains not less than 98.0% and not more than 101.0% of C12H17ClN4OS,HCl, calculated with reference to the dried substance.
Identity tests
A. Dissolve 10 mg in 1 mL of water, add 1 mL of sodium hydroxide (~80 g/l) TS and 0.5 mL of potassium ferricyanide (10 g/l) TS; the solution remains pale yellow.
Shake with 5 mL of 2-butanol R and allow to stand for 5-10 minutes; in bright daylight or in ultraviolet light (365 nm) the 2-butanol layer shows a blue fluorescence.
B. Spread a small quantity of the powder on a watch-glass; the odour is slight and characteristic, resembling that of yeast.
C. A 0.05 g/mL solution yields reaction A described under 2.1 General identification tests as characteristic of chlorides.
Heavy metals. Use 1.0 g for the preparation of the test solution as described under 2.2.3 Limit test for heavy metals, Procedure 1; determine the heavy metals content according to Method A; not more than 20 μg/g.
Clarity and colour of solution. A solution of 2.0 g in 10 mL of water is clear and not more intensely coloured than standard colour solution Yw2 when compared as described under 1.11.1 Colour of liquids.
Sulfated ash. Not more than 1.0 mg/g.
Loss on drying. Dry to constant weight at 105°C; it loses not more than 50 mg/g.
pH value. pH of a 25 mg/mL solution, 2.7-3.3.
Assay. In order to avoid overheating in the reaction medium, mix thoroughly throughout the titration and stop the titration immediately after the end-point has been reached.
Dissolve 0.110 g in 5 mL of anhydrous formic acid R and add 50 mL of acetic anhydride R. Carry out a potentiometric titration using perchloric acid (0.1 M) VS, as described 2.6 Non-aqueous titration. Perform the titration within 2 minutes and carry out a blank titration.
1 mL of perchloric acid (0.1 M) VS is equivalent to 16.86 mg of C12H17ClN4OS,HCl.