Monographs: Pharmaceutical substances: Trimethoprim (Trimethoprimum)
Molecular formula. C14H18N4O3
Relative molecular mass. 290.3
Graphic formula.
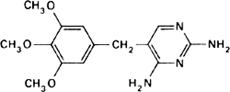
Chemical name. 2,4-Diamino-5-(3,4,5-trimethoxybenzyl)pyrimidine; 5-[(3,4,5-trimethoxyphenyl)methyl]-2,4-pyrimidinediamine; CAS Reg. No. 738-70-5.
Description. A white, crystalline powder; odourless or almost odourless.
Solubility. Sparingly soluble in water; soluble in methanol R; practically insoluble in ether R.
Category. Antibacterial.
Storage. Trimethoprim should be kept in a well-closed container.
Requirements
Definition. Trimethoprim contains not less than 98.5% and not more than 101.0% of C14H18N4O3, calculated with reference to the dried substance.
Identity tests
A. Carry out the examination as described under 1.7 Spectrophotometry in the infrared region. The infrared absorption spectrum is concordant with the spectrum obtained from trimethoprim RS or with the reference spectrum of trimethoprim.
B. Dissolve 25 mg in 5 mL of sulfuric acid (0.005 mol/l) VS, heat if necessary, and add 2 mL of a mixture of 1.6 g of potassium permanganate R dissolved in sufficient sodium hydroxide (0.1 mol/l) VS to produce 100 mL. Heat to boiling and add to the hot solution 0.4 mL of formaldehyde TS. Mix, add 1 mL of sulfuric acid (0.5 mol/l) VS, mix, and again heat to boiling. Cool to room temperature and filter. To the filtrate add 2 mL of chloroform R and shake the flask vigorously; a green fluorescence is produced in the chloroform layer when examined in ultraviolet light (365 nm).
C. Melting temperature, about 200°C.
Sulfated ash. Not more than 1.0 mg/g.
Loss on drying. Dry to constant weight at 105°C; it loses not more than 10 mg/g.
pH value. Shake 0.20 g with 20 mL of carbon-dioxide-free water R for 1 minute and filter; pH of the filtrate, 7.5-8.5.
Related substances
• The operations must be performed in a well-ventilated hood.
Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography, using silica gel R2 as the coating substance and a mixture of 85 volumes of ethyl acetate R, 10 volumes of methanol R, 5 volumes of water, and 2 volumes of anhydrous formic acid R as the mobile phase; an unlined chromatographic chamber should be used and the solvent front allowed to ascend 17 cm above the line of application. Apply separately to the plate 5 μl of each of 2 solutions in a mixture of 5 volumes of chloroform R, 4.5 volumes of methanol R, and 1 volume of water containing (A) 40 mg of the test substance per mL and (B) 0.080 mg of the test substance per mL. Pour the mobile phase into the chamber and insert the plate immediately so as to avoid prior saturation of the chamber. After removing the plate from the chromatographic chamber, allow it to dry in a stream of cold air for 5 minutes, and examine the chromatogram in ultraviolet light (254 nm). Place the plate in a closed chamber containing chlorine, produced by mixing equal volumes of a 15 mg/mL solution of potassium permanganate R and hydrochloric acid (~70 g/l) TS, placed at the bottom of the chamber, and allow to stand for 20 minutes. Remove the plate from the chamber and drive off the chlorine in a current of cold air until the area below the line of application does not give any blue colour on the addition of 0.05 mL of starch/iodide TS. Spray the plate with starch/iodide TS, and examine the chromatogram in daylight. Any spot obtained with solution A, other than the principal spot, is not more intense than that obtained with solution B.
Assay. Dissolve about 0.6 g, accurately weighed, in 30 mL of glacial acetic acid R1, and titrate with perchloric acid (0.1 mol/l) VS, as described under 2.6 Non-aqueous titration. Method A. Each mL of perchloric acid (0.1 mol/l) VS is equivalent to 29.03 mg of C14H18N4O3.
Additional requirement for Trimethoprim for parenteral use
Complies with the monograph for "Parenteral preparations".