Monographs: Pharmaceutical substances: Vinblastine sulfate (Vinblastini sulfas)
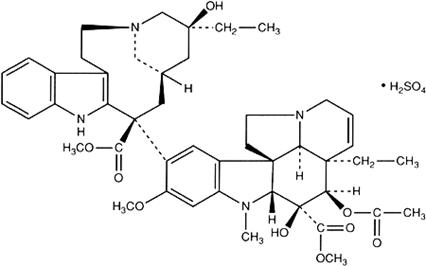
C46H58N4O9,H2SO4
Relative molecular mass. 909.1
Chemical name. Vincaleukoblastine sulfate (1:1) (salt); CAS Reg. No. 143-67-9.
Description. A white to slightly yellow, amorphous or crystalline powder.
Solubility. Freely soluble in water; very slightly soluble in ethanol (~750 g/l) TS; practically insoluble in ether R.
Category. Cytotoxic drug.
Storage. Vinblastine sulfate should be kept in a tightly closed container, protected from light, and stored at a temperature between 2 and 8 °C.
Additional information. CAUTION: Vinblastine sulfate must be handled with care, avoiding contact with the skin and inhalation of airborne particles. It is very hygroscopic and unstable. Before the bottle is opened, it should be allowed to come to room temperature in a desiccator.
Requirements
Vinblastine sulfate contains not less than 96.0% and not more than the equivalent of 101.0% of C46H58N4O9,H2SO4, calculated with reference to the dried substance.
Identity tests
• Either tests A and D or tests B, C, and D may be applied.
A. Carry out the examination as described under 1.7 Spectrophotometry in the infrared region. The infrared absorption spectrum is concordant with the spectrum obtained from vinblastine sulfate RS or with the reference spectrum of vinblastine sulfate.
B. See the test described below under "Related alkaloids". The principal spot obtained with solution A corresponds in position, appearance, and intensity with that obtained with solution C.
C. To 1 mg add 0.2 mL of vanillin/hydrochloric acid TS and allow to stand for 1 minute; a pink colour is produced (distinction from vincristine sulfate).
D. A 20 mg/mL solution yields reaction A described under 2.1 General identification tests as characteristic of sulfates.
Specific optical rotation. Use a 20 mg/mL solution in methanol R and calculate with reference to the dried substance;  = -28° to -35°.
= -28° to -35°.
Clarity of solution. A solution of 30 mg in 10 mL of water is clear.
Loss on drying. Dry at 60 °C under reduced pressure (not exceeding 0.6 kPa or 5 mm of mercury) for 16 hours; it loses not more than 170 mg/g.
pH value. pH of a 1.5 mg/mL solution, 3.5-5.0.
Related alkaloids. Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography, using silica gel R4 as the coating substance and a mixture of 80 volumes of toluene R, 40 volumes of chloroform R, and 6 volumes of diethylamine R as the mobile phase. Apply separately to the plate 5 μl of each of three solutions in methanol R containing (A) 10 mg of Vinblastine sulfate per mL, (B) 0.2 mg of vincristine sulfate RS per mL, and (C) 10 mg of vinblastine sulfate RS per mL. After removing the plate from the chromatographic chamber, allow it to dry in air, and examine the chromatogram in ultraviolet light (254 nm).
Any spot obtained with solution A, other than the principal spot, is not more intense than that obtained with solution B.
Assay. Dissolve about 10 mg, accurately weighed, in sufficient methanol R to produce 500 mL.
Measure the absorbance of this solution in a 1-cm layer at the maximum at about 267 nm. Calculate the content of C46H58N4O9,H2SO4, using the absorptivity value of 18.5 (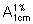 = 185).
= 185).