Monographs: Pharmaceutical substances: Benzyl benzoate (Benzylis benzoas)
Molecular formula. C14H12O2
Relative molecular mass. 212.3
Graphic formula.
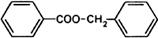
Chemical name. Phenylmethyl benzoate; CAS Reg. No. 120-51-4.
Description. A clear, colourless, oily liquid; odour, faintly aromatic.
Miscibility. Practically immiscible with water and glycerol R; miscible with ethanol (~750 g/l) TS and ether R.
Category. Scabicide (topical use).
Storage. Benzyl benzoate should be kept in a tightly closed and well-filled container, protected from light.
Additional information. Benzyl benzoate may slowly decompose on contact with air.
Requirements
Definition. Benzyl benzoate contains not less than 98.0% and not more than 100.5% of C14H12O2.
Identity tests
Boil 2 g with 25 mL of potassium hydroxide/ethanol TS2 for 10 minutes, evaporate the ethanol on a water-bath, cool, extract with 2 successive quantities, each of 15 mL of ether R, and proceed as follows:
A. Evaporate the ethereal layer on a water-bath; heat 1 drop of the oily liquid with 5 mL of sodium carbonate (50 g/l) TS and 1 mL of potassium permanganate (0.02 mol/l) VS; an odour of benzaldehyde is discernible.
B. To the aqueous layer add 10 mL of sulfuric acid (~100 g/l) TS; a white, crystalline precipitate is produced. Wash and dry the precipitate; melting temperature, about 123°C (benzoic acid).
Congealing temperature. Not below 17.0°C.
Refractive index. 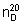 = 1.568 - 1.570
= 1.568 - 1.570
Mass density. ρ20 = 1.116 - 1.120 g/mL.
Chlorinated compounds. For the preparation of the test solution dissolve 0.30 g in 15 mL of ethanol (~750 g/l) TS and add 6 mL of sodium hydroxide (~80 g/l) TS. Warm the solution for 5 minutes on a water-bath. Cool, transfer to a comparison tube, and add 3 mL of nitric acid (~130 g/l) TS. For the reference solution transfer separately 2.0 mL of hydrochloric acid CITS and 4 mL of nitric acid (~130 g/l) TS to a comparison tube, and dilute to 25 mL with water. To both tubes add 0.5 mL of silver nitrate (40 g/l) TS. Stir immediately with a glass rod and set aside for 5 minutes, protected from direct sunlight. The opalescence produced from the test liquid is not stronger than that produced from the reference solution; not more than 0.33 mg/g of chlorine.
Acidity. Add 5 mL of the test liquid to 5 mL of neutralized ethanol TS, and titrate with carbonate-free sodium hydroxide (0.1 mol/l) VS, phenolphthalein/ethanol TS being used as indicator; not more than 0.3 mL is required to obtain the midpoint of the indicator (pink).
Assay. Add about 2.0 g, accurately weighed, to 40 mL of potassium hydroxide/ethanol (0.5 mol/l) VS and boil under reflux for 1 hour. Cool, and titrate with hydrochloric acid (0.5 mol/l) VS, using phenolphthalein/ethanol TS as indicator. Repeat the operation without the test liquid being examined and make any necessary corrections. Each mL of potassium hydroxide/ethanol (0.5 mol/l) VS is equivalent to 106.1 mg of C14H12O2.