Monographs: Dosage forms: General monographs: Powders for inhalation (Inhalanda)
2022-01
This text was drafted based on the corresponding texts in the chapters on Preparation of inhalation of the European Pharmacopoeia and the Japanese Pharmacopoeia. During the course of development, the text has undergone changes.
Definition
Powders for inhalation are solid preparations intended to be converted to aerosols and orally administered to the lung using a breath-actuated inhaler.
Powders for inhalation may be supplied in single-dose containers for use in pre-metered inhalers or in multidose containers for use in either pre-metered or device-metered powder inhalers. Pre-metered inhalers are loaded with powders pre-dispensed in capsules or other suitable dosage forms. Device-metered inhalers may use a powder reservoir where the dose is created by a metering mechanism within the inhaler.
Powders for inhalation contain one or more active pharmaceutical ingredients (API). To facilitate the use of powders for inhalation, the APIs may be combined with suitable excipients, for example, a carrier substance. These excipients do not adversely affect the functions of the mucosa of the respiratory tract or its cilia.
Additional information
The delivered dose is the dose delivered from the inhaler. For some preparations the labelled dose has been established as a metered dose or as a pre-metered dose. The metered dose is determined by adding the amount deposited on the inhaler to the delivered dose. It may also be determined directly.
Manufacture
The manufacturing processes for powders for inhalation should meet the requirements of good manufacturing practices (GMP).
The fine-particle characteristics of the aerosol cloud generated by the powder for inhalation is controlled so that a consistent portion is deposited in the lung.
In the manufacture, packaging, storage and distribution of powders for inhalation, suitable measures are taken to ensure their microbial quality. Recommendations on this aspect are provided in the chapter titled Microbiological quality of non-sterile products: recommended acceptance criteria for pharmaceutical preparations in the supplementary information section.
For a multidose powder inhaler, uniformity of a delivered dose must be ensured within a device (intra-inhaler) and between devices (inter-inhaler). For intra-inhaler testing, the uniformity of delivered dose tests are described below. For inter-inhaler testing, an example of a suitable procedure is to take 10 inhalers of a product and collect a single dose from each inhaler, collecting the dose at the beginning (from 3 inhalers), middle (from 4 inhalers) and end (from 3 inhalers) of the number of doses stated on the label. Other inter-inhaler testing procedures are possible, where justified and authorized.
Uniformity of delivered dose
Powders for inhalation comply with the following test.
The test is carried out using a dose collection apparatus that must be capable of quantitatively capturing the delivered dose. A suitable dose collection apparatus is specified in Figure 1 and Table 1.
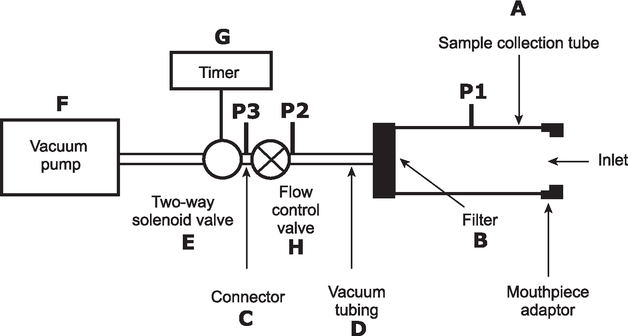
Figure 1. Apparatus suitable for measuring the uniformity of delivered dose for powder inhalers
Table 1. Specifications of the components of the apparatus used for powder inhalers described in Figure 1. Components from other manufacturers complying with the specifications may be employed.
|
Code |
Item |
Description |
|
A |
Sample collection tube |
Capable of quantitatively capturing the delivered dose, e.g. dose collection tube with dimensions of 34.85 mm ID × 12 cm length (e.g. product number XX40 047 00, Millipore Corporation, Bedford, MA 01732, USA, with modified exit tube, ID ≥ 8 mm, fitted with Gelman product number 61631), or equivalent. |
|
B |
Filter |
47 mm filter (e.g. A/E glass fibre filter, Gelman Sciences, Ann Arbor, MI 48106, USA), or equivalent. |
|
C |
Connector |
ID ≥ 8 mm, e.g., short metal coupling, with low-diameter branch to P3. |
|
D |
Vacuum tubing |
A length of suitable tubing having an ID ≥ 8 mm and an internal volume of 25 ± 5 mL. |
|
E |
2-way solenoid valve |
A 2-way, 2-port solenoid valve having a minimum airflow resistance orifice with ID ≥ 8 mm and an opening time ≤ 100 ms (e.g. type 256-A08, Bürkert GmbH, 74653 Ingelfingen, Deutschland), or equivalent. |
|
F |
Vacuum pump |
Pump must be capable of drawing the required flow rate through the assembled apparatus with the powder inhaler in the mouthpiece adapter (e.g. product type 1023, 1423 or 2565, Gast Manufacturing Inc., Benton Harbor, MI 49022, USA), or equivalent. Connect the pump to the 2-way solenoid valve using short and/or wide (≥ 10 mm ID) vacuum tubing and connectors to minimize pump capacity requirements. |
|
G |
Timer |
Timer capable of driving the 2-way solenoid valve for the required time period (e.g. type G814, RS Components International, Corby, NN17 9RS, UK), or equivalent. |
|
P1 |
Pressure tap |
2.2 mm ID, 3.1 mm OD, flush with internal surface of the sample collection tube, centred and burr-free, 59 mm from its inlet. The pressure tap P1 must never be open to the atmosphere. Differential pressure to atmosphere is measured at P1. |
|
P2 P3 |
Pressure measurements |
Absolute pressures. |
|
H |
Flow control valve |
Adjustable regulating valve with maximum Cv ≥ 1 (e.g. type 8FV12LNSS, Parker Hannifin plc., Barnstaple, EX31 1NP, UK), or equivalent. |
Unless otherwise stated, determine the test flow rate and duration using the dose collection tube, the associated flow system, a suitable differential pressure meter and a suitable volumetric flowmeter, calibrated for the flow leaving the meter, according to the following procedure.
Prepare the inhaler for use as directed in the instructions to the patient and connect it to the inlet of the apparatus using a mouthpiece adapter to ensure an airtight seal. Use a mouthpiece adapter that ensures that the front face of the inhaler mouthpiece is flush with the front face of the sample collection tube. Connect one port of a differential pressure meter to the pressure reading point P1 in Figure 1 and let the other be open to the atmosphere. Switch on the pump, open the 2-way solenoid valve and adjust the flow control valve until the pressure drop across the inhaler is 4.0 kPa (40.8 cm H2O) as indicated by the differential pressure meter. Remove the inhaler from the mouthpiece adapter and, without touching the flow control valve, connect a flowmeter to the inlet of the sampling apparatus. Use a flowmeter calibrated for the volumetric flow leaving the meter or calculate the volumetric flow leaving the meter (Qout) using the ideal gas law. For a meter calibrated for the entering volumetric flow (Qin), use the following expression:
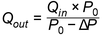
|
P 0 |
= |
atmospheric pressure; |
|
ΔP |
= |
pressure drop over the meter. |
If the flow rate is above 100 L/min, adjust the flow control valve to obtain a flow rate of 100 L/min (± 5 %). Note the volumetric airflow rate exiting the meter and define this as the test flow rate, Qout, in litres per minute. Define the test flow duration, T, in seconds so that a volume of 4 L of air is drawn from the mouthpiece of the inhaler at the test flow rate, Qout.
Ensure that critical flow occurs in the flow control valve by the following procedure: with the inhaler in place and the test flow rate Qout, measure the absolute pressure on both sides of the control valve (pressure reading points P2 and P3 in Figure 1); a ratio P3/P2 of less than or equal to 0.5 indicates critical flow; switch to a more powerful pump and re-measure the test flow rate if critical flow is not indicated.
Pre-metered inhalers. Connect the inhaler to the apparatus using an adapter that ensures a good seal. Draw air through the inhaler using the pre-determined conditions. Repeat the procedure until the number of deliveries that constitute the minimum recommended dose have been sampled. Quantitatively collect the contents of the apparatus and determine the amount of API. Repeat the procedure for a further 9 doses.
Device-metered inhalers. Connect the inhaler to the apparatus using an adapter that ensures a good seal. Draw air through the inhaler under the predetermined conditions. Repeat the procedure until the number of deliveries that constitute the minimum recommended dose have been sampled. Quantitatively collect the contents of the apparatus and determine the amount of API. Repeat the procedure for a further 2 doses.
Discharge the inhaler to waste until (n/2) + 1 deliveries remain, where n is the number of deliveries stated on the label. If necessary, store the inhaler to discharge electrostatic charges. Collect 4 doses using the procedure described above.
Discharge the inhaler to waste until 3 doses remain. If necessary, store the inhaler to discharge electrostatic charges. Collect 3 doses using the procedure described above.
For preparations containing more than 1 API, carry out the test for uniformity of delivered dose for each API.
Requirements. The preparation complies with the test if 9 out of 10 results lie between 75 % and 125 % of the mean value and all lie between 65 % and 135 %. If 2 or 3 values lie outside the limits of 75 % to 125 %, repeat the test for 2 more inhalers. Not more than 3 of the 30 values lie outside the limits of 75 % to 125 % and no value lies outside the limits of 65 % to 135 %.
If justified and authorized, these ranges may be extended but no value should be greater than 150 % or less than 50 % of the mean value. Unless otherwise authorized, the mean value must be between 85 % and 115 % of the label claim for delivered dose.
Fine particle dose
Using a suitable and authorized method, calculate the fine particle dose.
Number of deliveries per inhaler for multidose inhalers (this test may be combined with the test for Uniformity of delivered dose).
Using the method described in the test for Uniformity of delivered dose above, discharge doses from the inhaler until empty, at the predetermined flow rate. Record the deliveries discharged.
Requirements. The total number of deliveries so discharged from the inhaler is not less than the number stated on the label.
Storage
Powders for inhalation should be kept in well-closed containers and protected from moisture.
Labelling
Every finished pharmaceutical product must comply with the labelling requirements established under GMP.
The label should include:
(1) the name of the pharmaceutical product;
(2) the name(s) of the API(s); International Nonproprietary Names (INNs) should be used wherever possible;
(3) the amount of API in the delivered dose or, if justified and authorized (e.g. where the dose has been established as a metered dose or as a pre-metered dose), the metered dose or the pre-metered dose;
(4) where applicable, the number of deliveries from the inhaler to provide the minimum recommended dose;
(5) the number of deliveries per inhaler;
(6) the name and concentration of any antimicrobial preservative and the name of any other excipient;
(7) the batch (lot) number assigned by the manufacturer;
(8) the expiry date and, when required, the date of manufacture;
(9) any special storage conditions or handling precautions that may be necessary;
(10) directions for use, warnings, and precautions that may be necessary; and
(11) the name and address of the manufacturer or the person responsible for placing the product on the market.