Monographs: Dosage forms: Specific monographs: Streptomycin for injection (Streptomycini ad injectionem)
Description. A white or almost white powder.
Category. Antituberculosis drug.
Storage. Unless otherwise recommended by the manufacturer the reconstituted suspension should be used within 4 days when stored at a temperature between 2 and 8 °C and protected from light.
Labelling. The designation on the container should state the dose in the equivalent amount of streptomycin. It should also state the nature of the buffering agent, preservatives and stabilizers.
Additional information. Strength in the current WHO Model list of essential medicines: 1 g streptomycin (as sulfate). The injection is reconstituted by dilution of Streptomycin sulfate in Water for injections.
Requirements
The powder for injections and the reconstituted solution for injections comply with the monograph for Parenteral preparations.
Definition. Streptomycin for injection is a sterile powder of streptomycin sulfate. Streptomycin for injection may contain suitable buffers, preservatives and stabilizers. The powder is sterilized by a suitable method (see 5.8 Methods of sterilization).
Identity tests
- Either tests A and D or tests B, C and D may be applied.
A. Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography but using the coating prepared as follows: to 0.3 g of carbomer R add 240 mL of water, mix and shake moderately for 1 hour; then add gradually while stirring a sufficient volume of sodium hydroxide (~80 g/L) TS to adjust to pH 7.0. To this mixture add 30 g of silica gel R3 and coat the plate with a layer 0.75 mm thick. Heat the plate to 100 °C for 1 hour, cool and use immediately. Use potassium dihydrogen phosphate (70 g/L) TS as the mobile phase. Apply separately to the plate 10 μL of each of the following three solutions. For solution (A) dissolve a quantity of the powder for injections equivalent to 5 mg of Streptomycin sulfate in 5 mL of water. For solution (B) dissolve 10 mg of streptomycin sulfate RS in 10 mL of water. For solution (C) dissolve 1 mg of kanamycin monosulfate RS and 1 mg of framycetin sulfate RS in 1 mL of solution B. Develop the plate for a distance of 12 cm. After removing the plate from the chromatographic chamber allow it to dry in a current of warm air and spray with a mixture of equal volumes of naphthalene-1,3-diol/ethanol TS and sulfuric acid (~635 g/L) TS. Heat the plate to 150 °C for 5–10 minutes and examine the chromatogram in daylight.
The principal spot obtained with solution (A) corresponds in position, appearance and intensity with that obtained with solution (B). The test is valid only if the chromatogram obtained with solution (C) shows three clearly separated spots.
B. Dissolve a quantity of the powder for injections equivalent to 8 mg of Streptomycin sulfate in 4 mL of water, add 1 mL of sodium hydroxide (~40 g/L) TS and heat on a water-bath for 4 minutes. Cool and add 1 mL of hydrochloric acid (~70 g/L) TS and 0.1 mL of ferric chloride (63 g/L) TS; a violet colour is produced.
C. Dissolve a quantity of the powder for injections equivalent to 0.1 g of Streptomycin sulfate in 2 mL of water, add 1 mL of 1-naphthol TS1 and 2 mL of a mixture of equal volumes of sodium hypochlorite (~40 g/L) TS and water; a red colour is produced.
D. A solution containing a quantity of the powder for injections equivalent to 50 mg of Streptomycin sulfate yields reaction A described under 2.1 General identification tests as characteristic of sulfates.
Clarity of solution. A solution of the powder for injections equivalent to 2.5 g of Streptomycin sulfate in 10 mL of carbon-dioxide-free water R is clear. (Keep this solution for the "pH value".)
Loss on drying. Dry the powder for injections at 60 °C under reduced pressure (not exceeding 0.6 kPa or 5 mm of mercury) for 3 hours; it loses not more than 70 mg/g.
pH value. pH of the solution prepared above for the test of clarity and colour, 5.0–8.0.
Assays
Mix the contents of 10 containers and carry out the assays as described.
Potency. Carry out the assay as described under 3.1 Microbiological assay of antibiotics using either (a) Bacillus subtilis (NCTC 8236, or ATCC 11774) as the test organism, culture medium Cm1 with a final pH of 7.9–8.0, sterile phosphate buffer, pH 8.0, TS1 or TS2, an appropriate concentration of streptomycin (usually between 5 and 20 IU/mL), and an incubation temperature of 36–39 °C, or (b) Bacillus subtilis (ATCC 6633) as the test organism, culture medium Cm1 with a final pH of 8.0–8.1, sterile phosphate buffer, pH 8.0, TS1 or TS2, an appropriate concentration of streptomycin (usually between 3 and 15 IU/mL) and an incubation temperature of 35-37 °C. The precision of the assay is such that the fiducial limits of error of the estimated potency (P = 0.95) are not less than 95% and not more than 105% of the estimated potency.
The upper fiducial limit of error is not less than 720 IU per mg calculated with reference to the dried substance. Using the average contents and estimated potency calculate the total number of units of streptomycin in the container.
In places where the microbiological determination is not feasible perform the following alternative assay.
Streptomycin sulfate. Dissolve a quantity of the powder for injections equivalent to about 0.1 g of Streptomycin sulfate, accurately weighed, in sufficient water to produce 100 mL. To 5 mL add 5 mL of sodium hydroxide (0.2 mol/L) VS and heat in a water-bath for exactly 10 minutes. Cool in ice for exactly 5 minutes. Add 3 mL of ferric ammonium sulfate TS2 as well as sufficient water to produce 25 mL, mix and allow to stand for exactly 20 minutes after the addition of the last reagent.
Immediately measure the absorbance of a 1 cm layer at the maximum at about 525 nm against a solvent cell containing a solution prepared in the same manner but omitting the powder for injections being examined. Calculate the content of (C21H39N7O12)2,3H2SO4 in the powder for injections using the absorptivity value of 1.18 ( 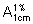 = 11.8). Using the result and the average mass of contents calculate the total content of streptomycin in the container using the conversion 800 mg of streptomycin being equivalent to 1 g of Streptomycin sulfate. The container of Streptomycin sulfate powder for injections contains not less than 90.0% and not more than 115.0% of the amount of C21H39N7O12 stated on the label.
= 11.8). Using the result and the average mass of contents calculate the total content of streptomycin in the container using the conversion 800 mg of streptomycin being equivalent to 1 g of Streptomycin sulfate. The container of Streptomycin sulfate powder for injections contains not less than 90.0% and not more than 115.0% of the amount of C21H39N7O12 stated on the label.
Bacterial endotoxins. Carry out the test as described under 3.4 Test for bacterial endotoxins; contains not more than 0.25 IU of endotoxin per mg of streptomycin.