Monographs: Dosage forms: Specific monographs: Zidovudine, lamivudine and abacavir tablets (Zidovudini, lamivudini et abacaviri compressi)
Category. Antiretroviral (Nucleoside Reverse Transcriptase Inhibitors).
Storage. Zidovudine, lamivudine and abacavir tablets should be kept in a tightly closed container, protected from light.
Labelling. The designation on the container of zidovudine, lamivudine and abacavir tablets should state that abacavir is in the sulphate form and the quantity should be indicated in terms of the equivalent amount of abacavir.
Additional information. Available strength: 300 mg Zidovudine, 150 mg Lamivudine and 300 mg abacavir.
The tablets are usually film-coated.
Requirements
Comply with the monograph for Tablets.
Definition. Zidovudine, lamivudine and abacavir tablets contain Zidovudine, Lamivudine and Abacavir sulfate. They contain not less than 90.0% and not more than 110.0% of the amounts of zidovudine (C10H13N5O4), lamivudine (C8H11N3O3S) and abacavir (C14H18N6O) stated on the label.
Identity tests
- Either test A or B may be applied.
A. Carry out test A.1 or, where UV detection is not available, test A.2.
A.1 Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography using silica gel R6 as the coating substance and a mixture of 90 volumes of dichloromethane R, 10 volumes of methanol R and 3 volumes of acetic acid R as the mobile phase. Apply separately to the plate 10 μl of each of the following 4 solutions in methanol R: (A) 3 mg of zidovudine RS per mL; (B) 1.5 mg of lamivudine RS per mL; and (C) 3.5 mg of abacavir sulfate RS per mL.
For solution (D) shake a quantity of the powdered tablets containing about 15 mg of Lamivudine (about 30 mg of Zidovudine and the equivalent of about 30 mg of abacavir) with 10 mL of methanol R, filter and use the filtrate. After removing the plate from the chromatographic chamber allow it to dry in a current of air and examine the chromatogram in ultraviolet light (254 nm).
The three principal spots obtained with solution D correspond in position, appearance and intensity to those obtained with solutions A, B and C.
A.2. Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography using the conditions given above under test A.1, but using silica gel R5 as the coating substance.
After removing the plate from the chromatographic chamber and allowing it to dry in a current of air. spray with vanillin/sulphuric acid TS1. Heat the plate for a few minutes at 120 °C. Examine the chromatogram in daylight.
The three principal spots obtained with solution D correspond in position, appearance and intensity to those obtained with solutions A, B and C.
B. See the test described below under “Assay”. The retention times of the three principal peaks in the chromatogram obtained with solution (1) are similar to those obtained with solution (2).
Related substances
Carry out the test as described under 1.14.1 Chromatography, High-performance liquid chromatography using the chromatographic conditions as described under “Assay”.
Prepare the following solutions in mobile phase A. For solution (1) transfer a quantity of the powdered tablets containing about 15 mg of Lamivudine (30 mg of Zidovudine and the equivalent of about 30 mg of abacavir) to a 100 mL volumetric flask, add 80 mL of mobile phase A, sonicate for 5 min and make up to volume with the same solvent. Filter through a 0.45 µm filter, discarding the first few mL of the filtrate. For solution (2) dilute 1.0 mL of solution (1) to 100.0 mL with mobile phase A. For solution (3) dissolve a small amount (about 2 mg) each of cytosine R, uracil R, thymine R, thymidine R and zidovudine impurity B RS in 10 mL of mobile phase A. Transfer 1.0 mL of this solution into a 100 mL volumetric flask and make up to volume with solution (1).
In the chromatogram obtained with solution (3) the three principal peaks elute in the order lamivudine (retention time about 12.5 minutes), zidovudine (retention time about 25 minutes) and abacavir (retention time about 31 minutes) and the following peaks are eluted at the following relative retention: with reference to lamivudine, lamivudine impurity E (cytosine) about 0.19; lamivudine impurity F (uracil) about 0.23: with reference to zidovudine, zidovudine impurity C (thymine) about 0.23; thymidine about 0.53; zidovudine impurity B about 1.06.
The test is not valid unless in the chromatogram obtained with solution (3) the resolution between cytosine and uracil is at least 4, the resolution between zidovudine and zidovudine impurity B is at least 3 and the peak-to-valley ratio between lamivudine and thymidine is at least 25.
In the chromatogram obtained with solution (1) the area of any peak corresponding to thymine, when multiplied by a correction factor of 0.6, is not more than twice the area of the principal peak due to zidovudine in the chromatogram obtained with solution (2) (2% with reference to zidovudine); the area of any peak eluting between those, if any, corresponding to uracil and thymine is not more than 0.3 times the area of the principal peak due to lamivudine in the chromatogram obtained with solution (2) (0.3 % with reference to lamivudine) and the area of any peak eluting between those corresponding to lamivudine and zidovudine, with the exception of the peak, if any, corresponding to thymidine is not more than 0.3 times the area of the principal peak due to abacavir in the chromatogram obtained with solution (2) (0.3 % with reference to abacavir).
Assay
Weigh and powder 20 tablets. Carry out the test under 1.14.1 Chromatography, High-performance liquid chromatography using a stainless steel column (15 cm × 4.6 mm) packed with particles of silica gel the surface of which has been modified with chemically-bonded octadecylsilyl groups (3.5 μm).
As the mobile phase use the following solutions:
Mobile phase A: ammonium acetate buffer pH 3.9.
Mobile phase B: methanol R.
Mobile phase C: acetonitrile R.
Prepare the ammonium buffer pH 3.9 by dissolving 1.9 g of ammonium acetate in 900 mL of water R. Adjust to pH 3.9 by addition of glacial acetic acid. Dilute to 1000 mL with water R.
Use the following gradient elution:
|
Time |
Mobile phase A |
Mobile phase B |
Mobile phase C |
Comments |
|
0–8 |
97 |
3 |
0 |
Isocratic |
|
8–19 |
97–76 |
3–24 |
0 |
Linear gradient |
|
19–27 |
76 |
24 |
0 |
Isocratic |
|
27–33 |
76–40 |
24–60 |
0 |
Linear gradient |
|
33–35 |
40 |
60 |
0 |
Isocratic |
|
35–35.1 |
40–0 |
60–0 |
0–100 |
Step gradient |
|
35.1–45 |
0 |
0 |
100 |
Washing of column |
|
45-45.1 |
0–97 |
0–3 |
100–0 |
Return to initial composition |
|
45.1–60 |
97 |
3 |
0 |
Re-equilibration |
Prepare the following solutions in mobile phase A. For solution (1) transfer a quantity of the powdered tablets containing about 15 mg of Lamivudine (30 mg of Zidovudine and the equivalent of about 30 mg of abacavir), accurately weighed, to a 100 mL volumetric flask, add 80 mL of mobile phase A, sonicate for 5 min and make up to volume with the same solvent. Filter through a 0.45 µm filter, discarding the first few mL of the filtrate. For solution (2) weigh 30 mg of zidovudine RS, 15 mg of lamivudine RS and 35 mg of abacavir sulfate RS in a 100 mL volumetric flask. Add approximately 80 mL of mobile phase A, sonicate until dissolved and dilute to volume. Solution (3) contains about 0.02 mg per mL of each of cytosine R, uracil R, thymine R, thymidine R and zidovudine impurity B RS in solution (2).
Operate with a flow rate of 0.7 mL per minute. As a detector use an ultraviolet spectrophotometer set at a wavelength of about 270 nm. Inject 10 µl of each solution.
In the chromatogram obtained with solution (3) the three principal peaks elute in the order lamivudine (retention time about 12.5 minutes), zidovudine (retention time about 25 minutes) and abacavir (retention time about 31 minutes) and the following peaks are eluted at the following relative retention: with reference to lamivudine, lamivudine impurity E (cytosine) about 0.19; lamivudine impurity F (uracil) about 0.23: with reference to zidovudine, zidovudine impurity C (thymine) about 0.23; thymidine about 0.53; zidovudine impurity B about 1.06.
The assay is not valid unless in the chromatogram obtained with solution (3) the resolution between cytosine and uracil is at least 4, the resolution between zidovudine and zidovudine impurity B is at least 3 and the peak to valley ratio between lamivudune and thymidine is at least 25.
Measure the areas of the peak responses in the chromatograms obtained with solutions (1) and (2) and calculate the content of zidovudine (C10H13N5O4), lamivudine (C8H11N3O3S) and abacavir (C14H18N6O) in the tablets, using the declared content of (C14H18N6O)2,H2SO4 in abacavir sulfate RS. Each mg of (C14H18N6O)2,H2SO4 is equivalent to 0.8537 mg of C14H18N6O .
Impurities
The impurities limited by the requirements of this monograph include:
impurity C (thymine) listed in the monograph for Zidovudine and the following zidovudine-related impurity
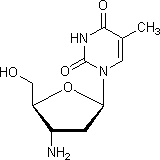
E. 1-(3-amino-2,3-dideoxy-β-D-erythro-pentofuranosyl)-5-methylpyrimidine-2,4(1H,3H)-dione (3'-amino-3'-deoxythimidine)
impurities E (cytosine), F (uracil), G and H listed in the monograph for Lamivudine;
impurity C listed in the monograph for Abacavir sulfate and the following abacavir-related impurity
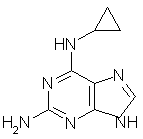
G. N6-cyclopropyl-1H-purine-2,6-diamine