Monographs: Dosage forms: Specific monographs: Estradiol valerate and norethisterone enantate injection (Estradioli valeras et norehtisteroni enantas injectio)
Description. A clear, colourless or almost colourless, oily solution.
Category. Contraceptive.
Storage. Estradiol valerate and norethisterone enantate injection should be kept in a closed container, protected from light.
Labelling. The oil used in the formulation should be indicated.
Additional information. Strength in the eighth invitation to manufacturers of reproductive health products to submit an expression of interest (EOI) for product evaluation to the World Health Organization (WHO) Prequalification Team – Medicines: 5 mg/mL of Estradiol valerate and 50 mg/ml of Norethisterone enantate in 1 mL ampoule.
Requirements
Definition. Estradiol valerate and norethisterone enantate injection contains not less than 90.0% and not more than 110.0% of the amounts of Estradiol valerate (C23H32O3) and Norethisterone enantate (C27H38O3) as stated on the label.
Identity tests
-
Test A and test B may be applied:
-
Carry out the test as described under 1.14.1 Chromatography, High-performance liquid chromatography, using the conditions as given under "Assay". The retention times of the peaks due to norethisterone enantate and estradiol valerate in the chromatogram obtained with solution (1) correspond to the retention times of the corresponding peaks in the chromatograms obtained with solutions (2) and (3).
-
Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography, using silica gel R6 or equivalent as the coating substance and a mixture of 4 volumes of cyclohexane R and 1 volume of ethyl acetate R as the mobile phase. Apply separately to the plate 5 µL of each of the following five solutions in methanol R. For solution (A), dilute 1.0 mL of the sample solution to 25.0 mL. For solution (B), dissolve 20 mg of norethisterone enantate RS and dilute to 10.0 mL. For solution (C), dissolve 2 mg of estradiol valerate RS and dilute to 10.0 mL. For solution (D), use a suitably diluted solution of the oil used in the formulation. For solution (E), dissolve 400 mg benzyl benzoate R in 25.0 mL. After removing the plate from the chromatographic chamber, allow it to air dry and examine the chromatogram in ultraviolet light (254 nm). Spray the plate with 4-toluenesulfonic acid/ethanol TS, heat at 120 °C for 10 minutes and examine the chromatogram in ultraviolet light (365 nm). The principal spots obtained with solution (A) corresponds in position and appearance to the spots due to norethisterone enantate obtained with solution (B) and due to estradiol valerate obtained with solution (C). The chromatogram of solution (A) may show spots due to the oil used in the formulation or benzyl benzoate.
Bacterial endotoxins. Carry out the test as described under 3.4 Test for bacterial endotoxins; contains not more than 1.5 IU of endotoxin RS per mg of norethisterone enantate.
Clarity and color of solution. Use 2.0 mL of the sample solution. This solution is clear and not more intensely coloured than reference solution Y3 or BY3 when compared as described under 1.11.2 Degree of coloration of liquids, Method I.
Related substances. Carry out the test as described under 1.14.1 Chromatography, High-performance liquid chromatography, using a stainless steel column (5 cm × 2.1 mm) packed with particles of silica gel, the surface of which has been modified with chemically-bonded octadecylsilyl groups (1.8 μm).
Use the following conditions for gradient elution:
-
mobile phase A: water R; and
-
mobile phase B: acetonitrile for chromatography R.
|
Time (minutes) |
Mobile phase A |
Mobile phase B |
Comments |
|
0–2 |
67 |
33 |
Isocratic |
|
2–17 |
67 to 35 |
33 to 65 |
Linear gradient |
|
17–25 |
35 to 0 |
65 to 100 |
Linear gradient |
|
25–30 |
0 |
100 |
Isocratic |
|
30–30.1 |
0 to 67 |
100 to 33 |
Return to initial composition |
|
30.1-33 |
67 |
33 |
Re-equilibration |
Operate with a flow rate of 0.8 mL per minute. As a detector, use an ultraviolet spectrophotometer set at a wavelength of 220 nm and 240 nm. Maintain the column temperature at 60 °C.
Prepare the following solutions using as the diluent a mixture of 15 volumes of water R and 85 volumes of acetonitrile R. For solution (1), use solution (1) as described under "Assay". For solution (2), dilute 5.0 mL of solution (1) to 500.0 ml. For solution (3), dilute 10.0 ml of this solution to 100.0 mL. For solution (4), prepare a solution of norethisterone enantate for system suitability RS (containing norethisterone and impurity F) as described in the leaflet of the reference substance. For solution (5), use a suitably diluted solution of the oil used in the formulation. For solution (6), dissolve 0.25g benzyl benzoate R in 100.0 mL.
Inject 5 μL each of solutions (2), (3) and (4) and record the chromatograms.
The test is not valid unless in the chromatogram obtained with solution (2), recorded at 220 nm, the signal-to-noise ratio of the peak due to estradiol valerate is at least 20. The test is also not valid unless in the chromatogram obtained with solution (3), recorded at 240 nm, the signal-to-noise ratio of the peak due to norethisterone enantate is at least 10. In the chromatogram obtained with solution (4), recorded at 240 nm, the resolution between the peak due to norethisterone enantate impurity F (norethisterone capreoate) and the peak due to norethisterone enantate is at least 3.0.
Inject alternately 10 µL each of solutions (1), (2), (5) and (6) and record the chromatograms.
Use the chromatogram obtained with solutions (5) and (6) to identify any peaks due to the oil used in the formulation and benzyl benzoate, if present. Estradiol valerate is eluted with a retention time of about 13.5 minutes and norethisterone enantate with a retention time of about 17 minutes.
In the chromatogram obtained with test solution (1), recorded at 220 nm:
-
the area of any impurity peak is not greater than 0.5 times the area of the peak due to estradiol valerate in the chromatogram obtained with solution (2), recorded at the same wavelength (0.5%).
In the chromatograms obtained with test solution (1), recorded at 240 nm:
-
the area of any impurity peak is not greater than 0.5 times the area of the peak due to norethisterone enantate in the chromatogram obtained with solution (2), recorded at the same wavelength (0.5%).
Assay. Carry out the test as described under 1.14.1 Chromatography, High-performance liquid chromatography, using the chromatographic conditions as described under "Related substances".
Prepare the following solutions using as the diluent a mixture of 15 volumes of water R and 85 volumes of acetonitrile R. Weigh the contents of 10 vials, mix and determine the weight per millilitre (1.3.1) of the solution. For solution (1), transfer a weighed quantity of the mixed contents, nominally equivalent to 75.0 mg of Norethisterone enantate, into a 250 mL volumetric flask. Add about 200 mL of the diluent, sonicate for 5 minutes, allow to cool to room temperature and dilute to volume. No oil droplets should be visible. For solution (2), dissolve 60.0 mg norethisterone enantate RS and dilute to 200.0 mL. For solution (3), dissolve 60.0 mg estradiol valerate RS and dilute to 200.0 ml. Dilute 10.0 mL of this solution to 100.0 mL.
Inject alternately 5 μL each of solutions (1), (2) and (3) and record the chromatograms.
Measure the areas of the peaks corresponding to norestisterone enantate obtained in the chromatograms of solutions (1) and (2), recorded at 240 nm, and calculate the percentage content of C27H38O3 per mL of the injection solution, using the declared content of C27H38O3 in norethisterone enantate RS. Measure the areas of the peaks corresponding to estradiol valerate obtained in the chromatograms of solutions (1) and (3), recorded at 220 nm, and calculate the percentage content of C23H32O3 per mL of the injection solution, using the declared content of C23H32O3 in estradiol valerate RS.
Impurities: The impurities limited by the requirements of this monograph include those listed in the monographs on Norethisterone enantate and on Estradiol valerate and the following:
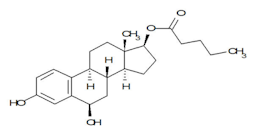
1. 3,6β-Dihydroxyestra-1,3,5(10)-trien-17β-yl-pentanoate (degradation product)
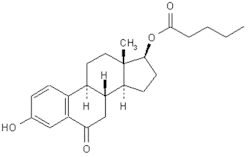
2. 3-Hydroxy-17β-valeryloxyestra-1,3,5(10)-trien-6-one (degradation product)