Monographs: Dosage forms: Specific monographs: Flucytosine intravenous infusion (Flucytosini infusio intraveno)
Description. Flucytosine intravenous infusion is a clear, colourless or almost colourless solution.
Category. Antifungal.
Storage. Flucytosine intravenous infusion should be kept in a tightly-closed container, protected from light.
Additional information. Strengths in the current WHO Model List of Essential Medicines (EML): 2.5 g in 250 mL. Strengths in the current EML for children: 2.5 g in 250 mL.
Requirements
Comply with the monograph for Parenteral preparations.
Definition. Flucytosine intravenous infusion is a sterile solution containing Flucytosine. It is supplied as a ready-to-use solution.
Flucytosine intravenous infusion contains not less than 90.0% and not more than 110.0% of the amount of Flucytosine (C4H4FN 3O) stated on the label.
Identity tests
- Either test A aloneor tests B and C may be applied.
A. Evaporate 10 mL of the infusion to dryness on a water-bath and dry the residue at 105 °C for about 1 hour. Carry out the examination as described under 1.7 Spectrophotometry in the infrared region. The infrared absorption spectrum is concordant with the spectrum obtained from flucytosine RS or with the reference spectrum of flucytosine.
B. Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography using silica gel R6 as the coating substance and a mixture of 60 volumes of nitromethane R, 20 volumes of methanol R, 10 volumes of ethyl acetate R and 10 volumes of water R as the mobile phase. Apply separately to the plate 1 μL of each of the following two solutions. Use a mixture composed of 60 volumes of methanol R, 35 volumes of water R and 5 volumes of glacial acetic acid R as the solvent. For solution (A) use an aliquot of the infusion to be tested. For solution (B) use 10 mg of flucytosine RS per mL. After application allow the spots to dry in a current of cool air. Develop over a path of 9 cm in an unsaturated chromatographic chamber. After removing the plate from the chromatographic chamber allow it to dry exhaustively in a current of air. Examine the chromatogram in ultraviolet light (254 nm). The principal spot obtained with solution (A) corresponds in position, appearance and intensity with that obtained with solution (B).
C. The absorption spectrum (1.6) of the final solution prepared for “Assay”, when observed between 230 nm and 350 nm, exhibits a maximum at about 286 nm and a minimum at about 245 nm.
pH value (1.13). pH of the infusion, 6.0–8.0.
Bacterial endotoxins. Carry out the test as described under 3.4 Test for bacterial endotoxins; contains not more than 0.1 IU of endotoxin per mg of flucytosine.
Related substances
Carry out the test as described under 1.14.1 Chromatography, High-performance liquid chromatography using a stainless steel column (25 cm x 4.0 mm) packed with end-capped particles of silica gel, the surface of which has been modified with chemically-bonded octadecylsilyl groups (5 μm).
As the mobile phase use a solution prepared as follows. Dissolve 13.6 g of potassium dihydrogen phosphate R in 950 mL of water R, adjust to pH 2.0 by adding phosphoric acid R and add 50 mL of methanol R.
Prepare the following solutions in a dissolution solvent prepared by dissolving 13.6 g of potassium dihydrogen phosphate R in 950 mL of water R and adding 50 mL of methanol R. For solution (1) dilute 3.0 ml of the infusion to 100.0 ml. For solution (2) dissolve 15.0 mg of fluorouracil RS (impurity A) in about 30 mL with sonication and dilute to 50.0 mL. Dilute 5.0 mL of this solution to 100.0 mL. Dilute 10.0 mL of this solution to 100.0 mL. For solution (3) dilute 1 volume of solution (1) to 200 volumes. Mix 1.0 mL of this solution with 1.0 mL solution (2).
Operate with a flow rate of 1.1 mL per minute. As a detector use an ultraviolet spectrophotometer set at a wavelength of about 260 nm.
Inject alternately 20 μL each of solution (1), (2) and (3) and record the chromatograms for 15 times the retention time of flucytosine.
Use the chromatogram obtained with solution (2) to identify the peak due to impurity A (fluorouracil). Flucytosine is eluted at a retention time about 2 minutes.
The test is not valid unless in the chromatogram obtained with solution (3) the resolution between the peaks due to flucytosine and impurity A (fluorouracil) is not less than 5.0 and the symmetry factor for the peak due to flucytosine is not more than 2.0.
In the chromatogram obtained with solution (1):
- the area of any peak due to impurity A (fluorouracil) is not greater than the area of the corresponding peak obtained with solution (2) (0.5%);
Assay
Dilute an accurately measured volume of the infusion with hydrochloric acid (0.1 mol/L) VS to give a solution containing about 0.1 mg per mL of Flucytosine. Dilute 5.0 mL of the resulting solution to 100.0 mL with the same solvent. Measure the absorbance of the resulting solution in a 1 cm layer at the maximum at about 286 nm. Calculate the content of Flucytosine (C4H4FN3O) using the absorptivity value of 70.9 ( 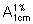 = 709).
= 709).
Impurities
The impurity limited by the requirements of this monograph is listed in the monograph for Flucytosine.