Monographs: Dosage forms: Specific monographs: Griseofulvin tablets (Griseofulvini compressi)
Category. Antifungal drug.
Labelling. Expiry date.
Additional information. Strength in the current WHO Model list of essential medicines: 125 mg, 250 mg.
Requirements
Comply with the monograph for "Tablets".
Griseofulvin tablets contain not less than 95.0% and not more than 105.0% of the amount of C17H17ClO6 stated on the label.
Identity tests
• Either test A alone or tests B and C may be applied.
A. Shake a quantity of the powdered tablets equivalent to 0.125 g of Griseofulvin with 20 mL of chloroform R and 1 g of anhydrous sodium sulfate R, and filter. Evaporate the filtrate to dryness and dry under reduced pressure (not exceeding 0.7 kPa) for 1 hour. Carry out the examination with the residue as described under 1.7 Spectrophotometry in the infrared region. The infrared absorption spectrum is concordant with the spectrum obtained from griseofulvin RS or with the reference spectrum of griseofulvin.
B. Carry out the test as described under 1.14.1 Chromatography, Thin-layer chromatography, using kieselguhr R1 as the coating substance and a mixture of 1 volume of ethylmethylketone R and 1 volume of xylene R as the mobile phase. Apply separately to the plate 10 μl of each of the following two solutions. For solution (A) shake a quantity of the powdered tablets equivalent to 5 mg of Griseofulvin with 10 mL of chloroform R, filter, and use the clear filtrate. For solution (B) dissolve 5 mg of griseofulvin RS in 10 mL of chloroform R. After removing the plate from the chromatographic chamber, allow it to dry in air, and examine the chromatogram in ultraviolet light (254 nm).
The principal spot obtained with solution A corresponds in position, appearance, and intensity with that obtained with solution B.
C. To a quantity of the powdered tablets equivalent to 5 mg of Griseofulvin add 1 mL of sulfuric acid (~1760 g/l) TS; a yellow-orange colour is produced. Add 1 drop of potassium dichromate (100 g/l) TS; the colour of the solution changes to wine-red.
Loss on drying. Dry a quantity of the powdered tablets equivalent to 0.1 g of Griseofulvin at 60 °C under reduced pressure (not exceeding 0.6 kPa or 5 mm of mercury) for 3 hours; it loses not more than 50 mg/g.
Assay. Weigh and powder 20 tablets. To a quantity of the powder equivalent to about 0.08 g of Griseofulvin, accurately weighed, add 150 mL of ethyl acetate R and boil under a reflux condenser for 15 minutes. Cool, add sufficient dehydrated ethanol R to produce 200 mL, shake, and centrifuge. Dilute 2.0 mL of the supernatant liquid to 100 mL with dehydrated ethanol R.
Measure the absorbance of this solution in a 1-cm layer at the maximum of about 291 nm and calculate the content of C17H17ClO6 using the absorptivity value of 68.6 (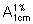 = 686).
= 686).
Dissolution. Carry out the test as described under 5.5 Dissolution test for solid oral dosage forms.